SAT - mvhs-fuhsd.org
... What Is Light? • Light is formed when electrons drop from the excited state to the ground state. • The lines on a bright-line spectrum come from specific energy level drops and are unique to each element. • Ex. Emission and Absorption Spectra ( line spectra) ...
... What Is Light? • Light is formed when electrons drop from the excited state to the ground state. • The lines on a bright-line spectrum come from specific energy level drops and are unique to each element. • Ex. Emission and Absorption Spectra ( line spectra) ...
Surface electrochemistry
... kurių metu išsiskiria energija. Short course annotation in English (up to 500 characters) The course presents the electrochemical processes on the surfaces making emphasis on adsorption, corrosion and oxidation. The other key topics include the study of the relationship between chemical change and e ...
... kurių metu išsiskiria energija. Short course annotation in English (up to 500 characters) The course presents the electrochemical processes on the surfaces making emphasis on adsorption, corrosion and oxidation. The other key topics include the study of the relationship between chemical change and e ...
Atomic Physics
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
2.8 M - Thierry Karsenti
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
down
... In principle, we should take linear combination of all the basis functions(basis set). However, we can reduce the number of AOs for which cij is nonzero by the energy of AO. Mixing between 1s and 2s : neglect in this level. Mixing between 2s and 2pz : both have σ symmetry → ‘s-p mixing’, but ...
... In principle, we should take linear combination of all the basis functions(basis set). However, we can reduce the number of AOs for which cij is nonzero by the energy of AO. Mixing between 1s and 2s : neglect in this level. Mixing between 2s and 2pz : both have σ symmetry → ‘s-p mixing’, but ...
Mathcad - CO Rotational States.
... peak is centered at ν J in a spectrum that has a range v. ∆ν is the width of the peak at half height. Each peak in a spectrum has a function like this to describe it. The full spectrum is obtained by plotting the Lorentian function as a function of v and J where J is the quantum number for the origi ...
... peak is centered at ν J in a spectrum that has a range v. ∆ν is the width of the peak at half height. Each peak in a spectrum has a function like this to describe it. The full spectrum is obtained by plotting the Lorentian function as a function of v and J where J is the quantum number for the origi ...
A Spectral Analysis of Laser Induced Fluorescence of Diatomic Iodine
... from the lowest vibrational state of the excited level. To understand why, note that at room temperature the electrons populate mostly the lowest vibrational levels of the ground state, indexed by the quantum number v 00 . Under incident visible light, the molecule will absorb photons and undergo vi ...
... from the lowest vibrational state of the excited level. To understand why, note that at room temperature the electrons populate mostly the lowest vibrational levels of the ground state, indexed by the quantum number v 00 . Under incident visible light, the molecule will absorb photons and undergo vi ...
Packet #6- Ionic and Covalent Bonding
... react with non-metals. Their ions have a single positive charge. For example, sodium forms sodium ions, Na+. The elements in Group 7 of the Periodic Table are called the halogens. They form ionic compounds when they react with metals. Their ions have a single negative charge. For example, chlorine f ...
... react with non-metals. Their ions have a single positive charge. For example, sodium forms sodium ions, Na+. The elements in Group 7 of the Periodic Table are called the halogens. They form ionic compounds when they react with metals. Their ions have a single negative charge. For example, chlorine f ...
Propagation of double Rydberg wave packets F Robicheaux and R C Forrey doi:10.1088/0953-4075/38/2/027
... There have been a few steps in the next obvious direction in which two electrons participate in the wave packet motion in a nontrivial manner [1–8]. In the early studies, both electrons are excited above the ground state although at least one of the electrons has been restricted to a quite small dis ...
... There have been a few steps in the next obvious direction in which two electrons participate in the wave packet motion in a nontrivial manner [1–8]. In the early studies, both electrons are excited above the ground state although at least one of the electrons has been restricted to a quite small dis ...
Document
... with its width. Well known highly durable novolak resist such as SAL ~chemically amplified negative resist for electron beam, SHIPLEY!, ZEP ~positive resist for electron beam, NIPPON ZEON!, and AZ ~photoresist, HOECHST! are based on phenol and contain benzene rings as the frame structure, and show h ...
... with its width. Well known highly durable novolak resist such as SAL ~chemically amplified negative resist for electron beam, SHIPLEY!, ZEP ~positive resist for electron beam, NIPPON ZEON!, and AZ ~photoresist, HOECHST! are based on phenol and contain benzene rings as the frame structure, and show h ...
Accurate van der Waals interactions from groundstate
... ● Analytical form known exactly for dispersion energy in HH ● Law of corresponding states for other spherical atoms (raregas, alkaline ...
... ● Analytical form known exactly for dispersion energy in HH ● Law of corresponding states for other spherical atoms (raregas, alkaline ...
Relativistic Quantum Mechanics
... We have found that the Klein-Gordon equation, a candidate for describing the quantum mechanics of spinless particles, admits unacceptable negative energy states when is interpreted as the single particle wave function. There is another way forward (this is the way followed in the textbook of Halze ...
... We have found that the Klein-Gordon equation, a candidate for describing the quantum mechanics of spinless particles, admits unacceptable negative energy states when is interpreted as the single particle wave function. There is another way forward (this is the way followed in the textbook of Halze ...
AP Chemistry
... When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same beaker. Yet another scale might report the mass as 249.89 g. W ...
... When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same beaker. Yet another scale might report the mass as 249.89 g. W ...
chemistry
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
Correlated many-electron states in a quantum dot containing a
... Here i1 / iN↑ and j1 / jN↓ are the indices of single states for which each index is set by two quantum numbers 共radial and azimuthal quantum numbers兲, as mentioned above. The number of possible configurations NC depends on the number of electrons, Ne, the number of single orbitals, NS, and the size ...
... Here i1 / iN↑ and j1 / jN↓ are the indices of single states for which each index is set by two quantum numbers 共radial and azimuthal quantum numbers兲, as mentioned above. The number of possible configurations NC depends on the number of electrons, Ne, the number of single orbitals, NS, and the size ...
Modern physics
... In order to avoid this, i.e. to have a localized particle with finite Δx we need a nearly definite momentum Δp≠0 . The narrower the width described by the weights A(p), the more precisely the momentum is constrained. The more precise the momentum, the more spread out the pulse is in space. The inver ...
... In order to avoid this, i.e. to have a localized particle with finite Δx we need a nearly definite momentum Δp≠0 . The narrower the width described by the weights A(p), the more precisely the momentum is constrained. The more precise the momentum, the more spread out the pulse is in space. The inver ...
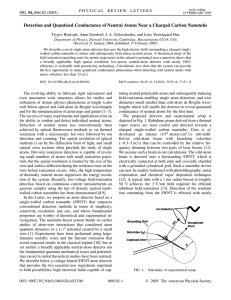
Detection and Quantized Conductance of Neutral Atoms Near a Charged... Trygve Ristroph, Anne Goodsell, J. A. Golovchenko, and Lene Vestergaard...
... tunneling electrons at the surface of the nanotube. The electron transmission coefficient through the tunneling barrier between the atom and the nanotube is most likely an oscillatory function of the radial distance between the atom and the nanotube. Thus we expect oscillations in the ion energy dis ...
... tunneling electrons at the surface of the nanotube. The electron transmission coefficient through the tunneling barrier between the atom and the nanotube is most likely an oscillatory function of the radial distance between the atom and the nanotube. Thus we expect oscillations in the ion energy dis ...
Bohr Theory in the Atomic Physics
... and that breaches the fact that the atom is quite stable. On the other hand, because the speed that the electron runs around the nucleus continuously change, so the wavelength of the atomic radiation will correspondingly continually change, and the spectrum emitted by the atom should be continuous s ...
... and that breaches the fact that the atom is quite stable. On the other hand, because the speed that the electron runs around the nucleus continuously change, so the wavelength of the atomic radiation will correspondingly continually change, and the spectrum emitted by the atom should be continuous s ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.