Answers to Selected Exercises
... its kinetic energy) drops to zero. Most of the kinetic energy is transferred to the sand, which deforms when the ball lands. Some energy is released as heat through friction between the ball and the sand. 4.11 The energy source of a 100-watt light bulb is electrical current from household wiring. En ...
... its kinetic energy) drops to zero. Most of the kinetic energy is transferred to the sand, which deforms when the ball lands. Some energy is released as heat through friction between the ball and the sand. 4.11 The energy source of a 100-watt light bulb is electrical current from household wiring. En ...
Notes for Quarter I
... Section 4 – Light and Color When light strikes any form of matter, it can interact with the matter in three different ways-it can be reflected, absorbed, or transmitted. Transmission is the passing of light through matter. Look at Figure 1, p. 652. When you look out of a window on a sunny day, you s ...
... Section 4 – Light and Color When light strikes any form of matter, it can interact with the matter in three different ways-it can be reflected, absorbed, or transmitted. Transmission is the passing of light through matter. Look at Figure 1, p. 652. When you look out of a window on a sunny day, you s ...
H 2 O
... • A priori, there should not be temperature effects at the level of the primary acts of radiolysis • Physical-chemical stage (ps): The temperature has an effect on the rate constants and on the diffusion constants: Arrhenius behavior The activation energies are low (10-20 kJ mol-1) ...
... • A priori, there should not be temperature effects at the level of the primary acts of radiolysis • Physical-chemical stage (ps): The temperature has an effect on the rate constants and on the diffusion constants: Arrhenius behavior The activation energies are low (10-20 kJ mol-1) ...
Solids and Light instructional units in one PDF fi
... is happening at the atomic level and use this model to understand LEDs. The LED is made up of a very small solid consisting of a large number of atoms which are closely packed together and interact with one another in a complex manner. When energy is supplied to the LED, these complex interactions r ...
... is happening at the atomic level and use this model to understand LEDs. The LED is made up of a very small solid consisting of a large number of atoms which are closely packed together and interact with one another in a complex manner. When energy is supplied to the LED, these complex interactions r ...
11 Two and many electron atoms - FU Berlin
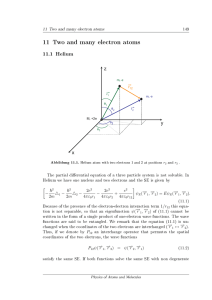
... than the singlet states, due to the reduced electron-electron repulsion. The triplet state (1s)(2s) is metastable, since relaxation to (1s)2 is not possible upon light emission (intercombination lines are forbidden). Excitation of triplet states is possible upon collisions of atoms, spin-orbit coupl ...
... than the singlet states, due to the reduced electron-electron repulsion. The triplet state (1s)(2s) is metastable, since relaxation to (1s)2 is not possible upon light emission (intercombination lines are forbidden). Excitation of triplet states is possible upon collisions of atoms, spin-orbit coupl ...
File
... _______17. How many moles are there in 3.40 grams of ammonia, NH 3 ? A) 57.8 B) 2.00 C) 5.00 D) 0.461 E) 0.200 _______18. 0.150 mole of propanone has a mass of 8.7 grams. What is the molar mass of propanone? (in grams/mol) A) 1.3 B) 17 C) 58 D) 8.7 _______19. Which of the following contains 3.01 x 1 ...
... _______17. How many moles are there in 3.40 grams of ammonia, NH 3 ? A) 57.8 B) 2.00 C) 5.00 D) 0.461 E) 0.200 _______18. 0.150 mole of propanone has a mass of 8.7 grams. What is the molar mass of propanone? (in grams/mol) A) 1.3 B) 17 C) 58 D) 8.7 _______19. Which of the following contains 3.01 x 1 ...
Chapter 28
... 55. The wave and particle characteristics of light are demonstrated a. in the photoelectric effect. b. in the Compton effect. c. in single slit diffraction. d. in double slit interference. e. only in separate experiments. ANS: e 56. When electrons interact with a double slit, a. each electron passes ...
... 55. The wave and particle characteristics of light are demonstrated a. in the photoelectric effect. b. in the Compton effect. c. in single slit diffraction. d. in double slit interference. e. only in separate experiments. ANS: e 56. When electrons interact with a double slit, a. each electron passes ...
Final Exam - KFUPM Faculty List
... In CO2 there are 2 CO σ-bonds, 2 CO π-bonds and 4 lone pairs, 2 on each oxygen. At each oxygen the σ-pair structure is formed by a triangle made up from the CO σ-bond and the 2 lone pairs. For these 3 electron pairs on each oxygen three hybrid orbitals are needed and thus an sp2 hybrid on each oxyge ...
... In CO2 there are 2 CO σ-bonds, 2 CO π-bonds and 4 lone pairs, 2 on each oxygen. At each oxygen the σ-pair structure is formed by a triangle made up from the CO σ-bond and the 2 lone pairs. For these 3 electron pairs on each oxygen three hybrid orbitals are needed and thus an sp2 hybrid on each oxyge ...
Surfaces, Interfaces, and Layered Devices
... Surface states emerge from the conduction and valence band since the total number of states is conserved. Surface states are usually partly filled, so the chemical potential is located within the surface band. Hence, the energy bands get bended and the Fermi level gets pinned – utmost important for ...
... Surface states emerge from the conduction and valence band since the total number of states is conserved. Surface states are usually partly filled, so the chemical potential is located within the surface band. Hence, the energy bands get bended and the Fermi level gets pinned – utmost important for ...
H - Deans Community High School
... The independent variable (what you changed), The dependent variable (what you measured), Safety The method mentioning all the equipment used and measurements made, readings and variable kept constant/changed etc 7. A table (with headings) of your measurements, and a sample average and rate = 1/t cal ...
... The independent variable (what you changed), The dependent variable (what you measured), Safety The method mentioning all the equipment used and measurements made, readings and variable kept constant/changed etc 7. A table (with headings) of your measurements, and a sample average and rate = 1/t cal ...
Full text in PDF - ndl nano
... fields, Coulomb interaction, and dielectric screening on electronic states and optical response of individual quantum dots are addressed in the literature in great detail.1–10 In a simplified picture, transport properties of arrays of weakly coupled quantum dots 共with wave functions well localized i ...
... fields, Coulomb interaction, and dielectric screening on electronic states and optical response of individual quantum dots are addressed in the literature in great detail.1–10 In a simplified picture, transport properties of arrays of weakly coupled quantum dots 共with wave functions well localized i ...
Quantum properties of spherical semiconductor quantum dots
... Semiconductor quantum dots (QDs), as well as quantum wires or quantum wells, show properties of standard atomic physics, as a result of the restriction of the motion of one to a hundred conduction band electrons or valence band holes to a confined region of space of nanometric size. But, in contrast ...
... Semiconductor quantum dots (QDs), as well as quantum wires or quantum wells, show properties of standard atomic physics, as a result of the restriction of the motion of one to a hundred conduction band electrons or valence band holes to a confined region of space of nanometric size. But, in contrast ...
Atomic Structure Institute of Lifelong Learning, University of Delhi
... Bohr was able to explain the stability of atoms as well as the emission spectrum of hydrogen with these postulates. However, soon it was realized that Bohr’s model of atom had many limitations and needed to be redefined. 1.2.1 Limitations of The Bohr’s Model of Atom 1. Bohr’s model of atom could not ...
... Bohr was able to explain the stability of atoms as well as the emission spectrum of hydrogen with these postulates. However, soon it was realized that Bohr’s model of atom had many limitations and needed to be redefined. 1.2.1 Limitations of The Bohr’s Model of Atom 1. Bohr’s model of atom could not ...
Quantum Monte Carlo study of the Ne atom and the Ne+ ion
... The “exact” nonrelativistic (NR) infinite-nuclear-mass energies are taken from Ref. [3]. All the DMC energies quoted in Table 1 have been extrapolated to zero time step. We used a range of small time steps and performed linear extrapolations of the energies to zero time step. Optimizing the orbitals ...
... The “exact” nonrelativistic (NR) infinite-nuclear-mass energies are taken from Ref. [3]. All the DMC energies quoted in Table 1 have been extrapolated to zero time step. We used a range of small time steps and performed linear extrapolations of the energies to zero time step. Optimizing the orbitals ...
103, 077001 (2009)
... new ingredients in the cuprate physics on a widely discussed transport coefficient—the Nernst coefficient—using a well-known model of the pseudogap—the ambipolar d-density-wave (DDW) model [11,12]—which is consistent with the findings of the quantum oscillation experiments. It will be shown that the ...
... new ingredients in the cuprate physics on a widely discussed transport coefficient—the Nernst coefficient—using a well-known model of the pseudogap—the ambipolar d-density-wave (DDW) model [11,12]—which is consistent with the findings of the quantum oscillation experiments. It will be shown that the ...
Electronic and magnetic properties of transition metal compounds
... Illustration of an XPS core level line [75]. . . . . . . . . . . . . . . . . Example for a chemical shift of the Fe 2p XPS core level lines of FeO. The spin-orbit coupling and satellites will be explained in Sections 2.3.2.4 and 2.3.2.5. . . . . . . . . . . . . . . . . . . . . . . . . . . . . Schema ...
... Illustration of an XPS core level line [75]. . . . . . . . . . . . . . . . . Example for a chemical shift of the Fe 2p XPS core level lines of FeO. The spin-orbit coupling and satellites will be explained in Sections 2.3.2.4 and 2.3.2.5. . . . . . . . . . . . . . . . . . . . . . . . . . . . . Schema ...
Pdf
... However, the quantum corrections to the classical limit arise largely from noncommutivity of kinetic and potential energy operators, so both operators must be present and explicit from the outset if one is to calculate these corrections. In the case of lattice gases consisting of structureless parti ...
... However, the quantum corrections to the classical limit arise largely from noncommutivity of kinetic and potential energy operators, so both operators must be present and explicit from the outset if one is to calculate these corrections. In the case of lattice gases consisting of structureless parti ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.