these notes as a Word document
... 1:1:2:3:5:8:13. Fibonacci, a fifteenth century Italian mathematician, discovered that as this additive integer series continues, it yields greater accuracy in defining an irrational ratio known as ‘the golden proportion.’ This proportion, which is found in the ratio of any two consecutive numbers in ...
... 1:1:2:3:5:8:13. Fibonacci, a fifteenth century Italian mathematician, discovered that as this additive integer series continues, it yields greater accuracy in defining an irrational ratio known as ‘the golden proportion.’ This proportion, which is found in the ratio of any two consecutive numbers in ...
Chapter 2
... a) atomic number b) atomic mass c) number of electrons d) number of protons e) none of the above 9. A particular carbon isotope has an atomic number of 6 and an atomic mass of 14. The respective number of neutrons, protons, and electrons that this carbon isotope has is _____. (Concept 2.2 ) a) 6, 8, ...
... a) atomic number b) atomic mass c) number of electrons d) number of protons e) none of the above 9. A particular carbon isotope has an atomic number of 6 and an atomic mass of 14. The respective number of neutrons, protons, and electrons that this carbon isotope has is _____. (Concept 2.2 ) a) 6, 8, ...
Problem Set II
... The second term in the expression provides the dependence of the energy on angular momentum. It is coupled to the first through the denominator that depends on r, the distance between the two bodies. a) Expand P2/2μr2 in a Taylor series in r about r = re. Perform the expansion through second order; ...
... The second term in the expression provides the dependence of the energy on angular momentum. It is coupled to the first through the denominator that depends on r, the distance between the two bodies. a) Expand P2/2μr2 in a Taylor series in r about r = re. Perform the expansion through second order; ...
Topic 3: Periodicity
... The M ion is the most stable for scandium to chromium. The M2+ ion is the most stable for Mn to Zn (the increased nuclear charge makes it more difficult to remove a third electron). In the higher oxidation states the elements usually not exist as a free metal ions, but covalently bonded or as a oxya ...
... The M ion is the most stable for scandium to chromium. The M2+ ion is the most stable for Mn to Zn (the increased nuclear charge makes it more difficult to remove a third electron). In the higher oxidation states the elements usually not exist as a free metal ions, but covalently bonded or as a oxya ...
Chemistry exam review
... 2. The gases helium, neon, and argon are in separate containers at 55°C. Which is true about the kinetic energy of the gases? a. Helium has the lowest mass and therefore the greatest kinetic energy. b. They each have a different kinetic energy. c. Argon has greatest mass and therefore the greatest ...
... 2. The gases helium, neon, and argon are in separate containers at 55°C. Which is true about the kinetic energy of the gases? a. Helium has the lowest mass and therefore the greatest kinetic energy. b. They each have a different kinetic energy. c. Argon has greatest mass and therefore the greatest ...
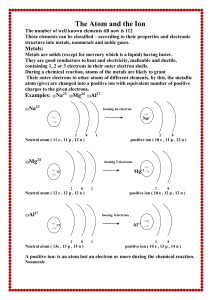
The Atom and the Ion
... liquid element which is bromine. They have no luster, not malleable or ductile (brittle), they are bad conductors to heat and electricity, except graphite which is good conductor to electricity. Most of nonmetals contain 5,6 or 7 electrons in their outer shells. Nonmetals atoms are likely to gain el ...
... liquid element which is bromine. They have no luster, not malleable or ductile (brittle), they are bad conductors to heat and electricity, except graphite which is good conductor to electricity. Most of nonmetals contain 5,6 or 7 electrons in their outer shells. Nonmetals atoms are likely to gain el ...
III. Quantum Model of the Atom
... • Relative Size of the orbital • n = # of sublevels in that energy level • n2 = # of orbitals in the energy level • 2n2 = total # of electrons in that energy level ...
... • Relative Size of the orbital • n = # of sublevels in that energy level • n2 = # of orbitals in the energy level • 2n2 = total # of electrons in that energy level ...
CH03_Tro_LectureNotes - Tutor
... that exist in nature as individual atoms include Na, C, Fe, Ca, etc. Some elements are more stable in nature as diatomic molecules (two atoms of the same type), such as O2, N2, Cl2, etc. In an element that exists naturally in the diatomic form, the two atoms are always identical. A compound is made ...
... that exist in nature as individual atoms include Na, C, Fe, Ca, etc. Some elements are more stable in nature as diatomic molecules (two atoms of the same type), such as O2, N2, Cl2, etc. In an element that exists naturally in the diatomic form, the two atoms are always identical. A compound is made ...
10. Molecules and Solids
... photons and make transitions to a higher vibrational state when electromagnetic radiation is incident upon a collection of a particular kind of molecule. ...
... photons and make transitions to a higher vibrational state when electromagnetic radiation is incident upon a collection of a particular kind of molecule. ...
Planck-Einstein relation, Time Dep. Schrodinger Eq., Po
... leap of analogy. First look at what the units of ∆ are; they are time−1 , i.e., the units of frequency. What physical observable has units of frequency? The Planck- Einstein relation says that E = h̄ω where ω is frequency and h̄ = h/2π, with h the fundamental Planck constant. So lets choose our oper ...
... leap of analogy. First look at what the units of ∆ are; they are time−1 , i.e., the units of frequency. What physical observable has units of frequency? The Planck- Einstein relation says that E = h̄ω where ω is frequency and h̄ = h/2π, with h the fundamental Planck constant. So lets choose our oper ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.