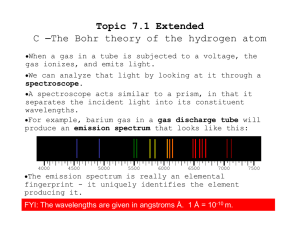
Chapter 4 Section 1 The Development of a New Atomic Model
... • The lowest energy state of an atom is its ground state. • A state in which an atom has a higher potential energy than it has in its ground state is an excited state. ...
... • The lowest energy state of an atom is its ground state. • A state in which an atom has a higher potential energy than it has in its ground state is an excited state. ...
CHEM-UA 127: Advanced General Chemistry I
... Finally, suppose we start with a state Ψ(x, 0) = (1/ 2)[ψ1 (x) + ψ2 (x)], and we let this state evolve in time. At any point in time, the state Ψ(x, t) will be some mixture of ψ1 (x) and ψ2 (x), and this mixture changes with time. Now, at some specific instance in time t, we measure the energy and o ...
... Finally, suppose we start with a state Ψ(x, 0) = (1/ 2)[ψ1 (x) + ψ2 (x)], and we let this state evolve in time. At any point in time, the state Ψ(x, t) will be some mixture of ψ1 (x) and ψ2 (x), and this mixture changes with time. Now, at some specific instance in time t, we measure the energy and o ...
50 Frequently Forgotten Facts Answer Key
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
A simple and effective approach to calculate the energy of complex
... is based on the Z −1 expansion due to Layzer [9] and originated in works of Hylleraas on the ground state of He. On one hand, this is an interesting exercise for nongraduate students. We show as to attain data even for complex atoms that are well compared with the experiment in diverse cases: bindin ...
... is based on the Z −1 expansion due to Layzer [9] and originated in works of Hylleraas on the ground state of He. On one hand, this is an interesting exercise for nongraduate students. We show as to attain data even for complex atoms that are well compared with the experiment in diverse cases: bindin ...
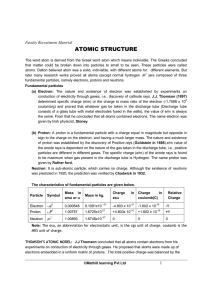
atomic structure
... which various subshells are filled up depending on the relative order of the energy of orbitals. “Electrons are added progressively to the various orbitals in the order of increasing energy starting with the orbital of lowest energy” ...
... which various subshells are filled up depending on the relative order of the energy of orbitals. “Electrons are added progressively to the various orbitals in the order of increasing energy starting with the orbital of lowest energy” ...
Review topics-blog
... scale in your bathroom might weight out to the nearest 1 pound or maybe to the nearest 0.1 pound. Most objects could be described by a much more precise mass than this, though this bathroom scale would be limited to only the placeholders shown on its display. Often you find tools this have some u ...
... scale in your bathroom might weight out to the nearest 1 pound or maybe to the nearest 0.1 pound. Most objects could be described by a much more precise mass than this, though this bathroom scale would be limited to only the placeholders shown on its display. Often you find tools this have some u ...
Chapter 5 - U of L Class Index
... elements in compounds are replaced by other elements. If only one compound has an element replaced, it is a single replacement reaction. If two compounds have elements replaced, then it is a double replacement reaction. e.g. Fe2O3 + 3C AgNO3 + NaCl ...
... elements in compounds are replaced by other elements. If only one compound has an element replaced, it is a single replacement reaction. If two compounds have elements replaced, then it is a double replacement reaction. e.g. Fe2O3 + 3C AgNO3 + NaCl ...
Electron±electron correlations in carbon nanotubes
... function of B. Because of the higher bias voltage, the trace has broadened into a stripe with additional structure due to excitedstate transitions. Along the B-axis, the stripe consists of four segments, of which the second and the third appear to have clearly different widths in Vgate. This is very ...
... function of B. Because of the higher bias voltage, the trace has broadened into a stripe with additional structure due to excitedstate transitions. Along the B-axis, the stripe consists of four segments, of which the second and the third appear to have clearly different widths in Vgate. This is very ...
Title Goes Here
... systems, this excitonic effect becomes strong due to its large quantum confinement [someya]. However, it has not been fully understood how the 1D excitonic effect is weakened by screening or phase-space-filling effects caused by the 1D electron gas. Our experiments provided a quantitative value of t ...
... systems, this excitonic effect becomes strong due to its large quantum confinement [someya]. However, it has not been fully understood how the 1D excitonic effect is weakened by screening or phase-space-filling effects caused by the 1D electron gas. Our experiments provided a quantitative value of t ...
Chapter 2
... An ionic bond can form if two atoms are so unequal in their attraction for valence electrons that one atom strips an electron completely from the other. o For example, sodium, with 1 valence electron in its third shell, transfers this electron to chlorine, with 7 valence electrons in its third shell ...
... An ionic bond can form if two atoms are so unequal in their attraction for valence electrons that one atom strips an electron completely from the other. o For example, sodium, with 1 valence electron in its third shell, transfers this electron to chlorine, with 7 valence electrons in its third shell ...
Electronic Structure and the Periodic Table
... Things get a bit more complex where more than one electron is involved. Effective nuclear charge(kernel charge) Inner electrons act to shield outer ones from the positive charge of the nucleus. Some orbitals penetrate to the nucleus more than others: s > p > d > f ...
... Things get a bit more complex where more than one electron is involved. Effective nuclear charge(kernel charge) Inner electrons act to shield outer ones from the positive charge of the nucleus. Some orbitals penetrate to the nucleus more than others: s > p > d > f ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.