PREPARATION, STRUCTURAL STUDIES AND CHEMICAL
... Likewise the concept of three-center, two-electron bond (3c-2e) has been developed for electron deficient compounds, the concept of three-center, four-electron bond (3c-4e) has been created to explain electronic structure of hypervalent compounds. The term "hypervalent" has been introduced by J. I. ...
... Likewise the concept of three-center, two-electron bond (3c-2e) has been developed for electron deficient compounds, the concept of three-center, four-electron bond (3c-4e) has been created to explain electronic structure of hypervalent compounds. The term "hypervalent" has been introduced by J. I. ...
AP Reactions - Georgetown ISD
... Rules for Assigning Oxidation States—you know most of this already! The oxidation state of an atom in an element is ZERO including allotropes [i.e. N2, P4, S8]. The oxidation state of a monatomic ion is the same as its charge. In its compounds, fluorine is always assigned an oxidation state of -1. O ...
... Rules for Assigning Oxidation States—you know most of this already! The oxidation state of an atom in an element is ZERO including allotropes [i.e. N2, P4, S8]. The oxidation state of a monatomic ion is the same as its charge. In its compounds, fluorine is always assigned an oxidation state of -1. O ...
AP Chemistry Note Outline
... 6. Cancel out any extra water and OH7. Balance Charge with e8. Multiply reactions by factors such that the e- cancel Add both ½ reactions ...
... 6. Cancel out any extra water and OH7. Balance Charge with e8. Multiply reactions by factors such that the e- cancel Add both ½ reactions ...
AP_chemical reaction and quantities
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
CHEMISTRY
... know the algebraic order of operations, so they tend to use their calculator incorrectly. 3. An understanding of metric prefixes and their meanings is crucial for conversions between units. I use dimensional analysis to do metric-metric conversions, stressing that many different types of calculation ...
... know the algebraic order of operations, so they tend to use their calculator incorrectly. 3. An understanding of metric prefixes and their meanings is crucial for conversions between units. I use dimensional analysis to do metric-metric conversions, stressing that many different types of calculation ...
A-level Chemistry Modified question paper Unit 01
... molecule of dodecane into equal amounts of two different molecules each containing the same number of carbon atoms. State the empirical formula of the straight-chain alkane that is formed. Name the catalyst used in this reaction. [3 marks] ...
... molecule of dodecane into equal amounts of two different molecules each containing the same number of carbon atoms. State the empirical formula of the straight-chain alkane that is formed. Name the catalyst used in this reaction. [3 marks] ...
231. - Department of Chemistry
... C5H5)Fe(H) ] [13] also have been synthesized and extensively investigated in the gas phase. In this study we report the completion of measurements of the kinetics and energetics of the ligation of (c-C5H5)Fe⫹ in He bath gas at 0.35 Torr with a variety of inorganic ligands containing hydrogen, carbon ...
... C5H5)Fe(H) ] [13] also have been synthesized and extensively investigated in the gas phase. In this study we report the completion of measurements of the kinetics and energetics of the ligation of (c-C5H5)Fe⫹ in He bath gas at 0.35 Torr with a variety of inorganic ligands containing hydrogen, carbon ...
Chapter 4 - profpaz.com
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
Energetics Past Paper Questions
... Separate solutions of HCl(aq) and H2SO4(aq) of the same concentration and same volume were completely neutralized by NaOH(aq). X kJ and Y kJ of heat were evolved respectively. Which statement is correct? ...
... Separate solutions of HCl(aq) and H2SO4(aq) of the same concentration and same volume were completely neutralized by NaOH(aq). X kJ and Y kJ of heat were evolved respectively. Which statement is correct? ...
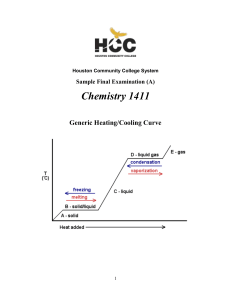
1411FINALSAMPLEs and Key
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
Document
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
Document
... Pure titanium is not rejected by the body and has been used in replacing missing human teeth with great success. Copper is used for cooking ware, electrical cables and water pipes. Iron is the most important structural metal ever used ...
... Pure titanium is not rejected by the body and has been used in replacing missing human teeth with great success. Copper is used for cooking ware, electrical cables and water pipes. Iron is the most important structural metal ever used ...
Deans Community High School Intermediate 2 Revision Notes www
... and products actually begin to decompose or react in different ways if the temperature is too high; so although the temperature gives the collisions enough energy to cause a chemical reaction, the product may decompose before it can be isolated. Catalysts reduce the energy required for a reaction to ...
... and products actually begin to decompose or react in different ways if the temperature is too high; so although the temperature gives the collisions enough energy to cause a chemical reaction, the product may decompose before it can be isolated. Catalysts reduce the energy required for a reaction to ...
Questions and Solutions
... Indicate which terms apply to each species. There is more than one term which applies to each species. N (neutral) ...
... Indicate which terms apply to each species. There is more than one term which applies to each species. N (neutral) ...
Final Exam Review Notes
... SCIENTIFIC NOTATION Some numbers are very large or very small difficult to express. For example, Avogadro’s number = 602,000,000,000,000,000,000,000 an electron’s mass = 0.000 000 000 000 000 000 000 000 000 91 kg Also, it's not clear how many sig figs there are in some measurements. For example, ...
... SCIENTIFIC NOTATION Some numbers are very large or very small difficult to express. For example, Avogadro’s number = 602,000,000,000,000,000,000,000 an electron’s mass = 0.000 000 000 000 000 000 000 000 000 91 kg Also, it's not clear how many sig figs there are in some measurements. For example, ...
physical setting chemistry
... (1) They have identical molecular and identical properties. (2) They have identical molecular and different properties. (3) They have different molecular and identical properties. (4) They have different molecular and different properties. ...
... (1) They have identical molecular and identical properties. (2) They have identical molecular and different properties. (3) They have different molecular and identical properties. (4) They have different molecular and different properties. ...
NYS Regents Chemistry
... hh. Metals and non-metals separated by “staircase” beginning at Group 13 i. Metals to the left of the “staircase” (except H) (most elements are metals) ii. Non-metals to the right of the “staircase” (including H) ii. Properties of Metals: i. Are mostly solids (one liquid, Hg) ii. Lose electrons easi ...
... hh. Metals and non-metals separated by “staircase” beginning at Group 13 i. Metals to the left of the “staircase” (except H) (most elements are metals) ii. Non-metals to the right of the “staircase” (including H) ii. Properties of Metals: i. Are mostly solids (one liquid, Hg) ii. Lose electrons easi ...
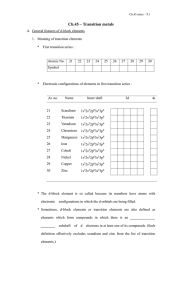
Deformations from Octahedral Geometry in d4 Transition
... the octahedral parentage would have led to an excursion along a ta or eg~ibrati0n.l~ What we found impressive about compounds 4-6 was that their deformation from octahedral symmetry was (a) substantial, (b) varied-here toward a bicapped tetrahedron, there toward a trigonal prism with carbonyls movin ...
... the octahedral parentage would have led to an excursion along a ta or eg~ibrati0n.l~ What we found impressive about compounds 4-6 was that their deformation from octahedral symmetry was (a) substantial, (b) varied-here toward a bicapped tetrahedron, there toward a trigonal prism with carbonyls movin ...
Dr. Baxley`s Thermodynamics Worksheet
... d) PF3(g) < PF5(g) < PF2Cl3(g) 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous reactants than products. ∆H is − because this is a combustion ...
... d) PF3(g) < PF5(g) < PF2Cl3(g) 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous reactants than products. ∆H is − because this is a combustion ...
Notes-C12-121
... Organic and Inorganic Compounds Organic vs. Inorganic • Organic chemistry: Study of hydrocarbons (only carbon and hydrogen atoms) and their various derivatives. – Examples: natural gas, petroleum, plastics, rubbers, paper, carbohydrates (sugar, starch), proteins, enzymes, fatty acids, food stuff, dr ...
... Organic and Inorganic Compounds Organic vs. Inorganic • Organic chemistry: Study of hydrocarbons (only carbon and hydrogen atoms) and their various derivatives. – Examples: natural gas, petroleum, plastics, rubbers, paper, carbohydrates (sugar, starch), proteins, enzymes, fatty acids, food stuff, dr ...
Chemistry Notes for the Whole Year Powerpoint
... • Three rules govern electron configurations and they are: • Aufbau principle-electrons enter orbitals of lowest energy first. For all electron configurations, start filling electrons in the 1s orbital, which is the lowest energy level. • Pauli exclusion principle-An atomic orbital may describe at m ...
... • Three rules govern electron configurations and they are: • Aufbau principle-electrons enter orbitals of lowest energy first. For all electron configurations, start filling electrons in the 1s orbital, which is the lowest energy level. • Pauli exclusion principle-An atomic orbital may describe at m ...