Physical Sciences Grade 10 Term 2
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
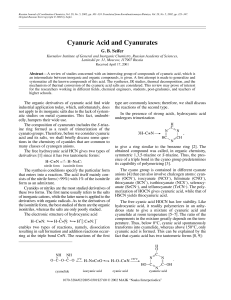
Cyanuric Acid and Cyanurates
... The entropy changes and the heat effect of the HCN polymerization are calculated in [22], while the magnetic anisotropy and the charge delocalization in S-triazine are considered in [23]. The IR spectrum of the polymerized HCN is given in [3] (ν, cm–1): 3450, 3370, 3314, 3260, 3219, 3184 ν(NH2); 222 ...
... The entropy changes and the heat effect of the HCN polymerization are calculated in [22], while the magnetic anisotropy and the charge delocalization in S-triazine are considered in [23]. The IR spectrum of the polymerized HCN is given in [3] (ν, cm–1): 3450, 3370, 3314, 3260, 3219, 3184 ν(NH2); 222 ...
Rhenium- and molybdenum-catalyzed dehydration reactions
... derfunctionalized, lignocellulosic biomass-based compounds have an O : C ratio close to one and are highly functionalized with hydroxyl groups. Therefore a completely different type of chemistry is required to acquire building blocks from lignocellulosic biomass suitable for the chemical industry: ...
... derfunctionalized, lignocellulosic biomass-based compounds have an O : C ratio close to one and are highly functionalized with hydroxyl groups. Therefore a completely different type of chemistry is required to acquire building blocks from lignocellulosic biomass suitable for the chemical industry: ...
Soln Chem 2008Nov(9746)
... E o value becomes less positive suggests a decrease in oxidising power : X2 > Y2 > Z2. ...
... E o value becomes less positive suggests a decrease in oxidising power : X2 > Y2 > Z2. ...
Reaction Rates
... When the orientation of colliding molecules is correct, as shown in Figure 4c, a reaction can occur. An oxygen atom is transferred from an NO 2 molecule to a CO molecule. When this occurs, a short-lived entity called an activated complex is formed, in this case OCONO. An activated complex, sometimes ...
... When the orientation of colliding molecules is correct, as shown in Figure 4c, a reaction can occur. An oxygen atom is transferred from an NO 2 molecule to a CO molecule. When this occurs, a short-lived entity called an activated complex is formed, in this case OCONO. An activated complex, sometimes ...
Chapter 16: Reaction Rates
... When the orientation of colliding molecules is correct, as shown in Figure 16.4c, a reaction can occur. An oxygen atom is transferred from an NO 2 molecule to a CO molecule. When this occurs, a short-lived entity called an activated complex is formed, in this case OCONO. An activated complex, someti ...
... When the orientation of colliding molecules is correct, as shown in Figure 16.4c, a reaction can occur. An oxygen atom is transferred from an NO 2 molecule to a CO molecule. When this occurs, a short-lived entity called an activated complex is formed, in this case OCONO. An activated complex, someti ...
Problem 1-2
... Rate law constants can be determined by the method of relaxation. Thereby the system which is already in equilibrium is disturbed e.g. by a sudden change of temperature. Then rate constants can be determined by the observation of the adjustment of the new ...
... Rate law constants can be determined by the method of relaxation. Thereby the system which is already in equilibrium is disturbed e.g. by a sudden change of temperature. Then rate constants can be determined by the observation of the adjustment of the new ...
Changing Matter
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... • homogeneous mixtures are called solutions • the component of the solution that changes state is called the solute • the component that keeps its state is called the solvent – if both components start in the same state, the major component is the solvent Burns 4/e Chap 4 ...
... • homogeneous mixtures are called solutions • the component of the solution that changes state is called the solute • the component that keeps its state is called the solvent – if both components start in the same state, the major component is the solvent Burns 4/e Chap 4 ...
BSc Honours chemistry CBCS Syllabus 2016-17
... (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals.Bent’s rule, Resonance and resonance energy, Molecular orbital theory. Molecular orbital diagrams of diatomic and simple polyatomic molecules ...
... (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals.Bent’s rule, Resonance and resonance energy, Molecular orbital theory. Molecular orbital diagrams of diatomic and simple polyatomic molecules ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Q3- What is a pure substance? (L- 1 ) Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L- ...
... Q3- What is a pure substance? (L- 1 ) Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L- ...
Chapter 6 Quantities in Chemical Reactions
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
Solving General Chemistry Problems 5e
... Number Notations, Arithmetical Operations, and Calculators ...
... Number Notations, Arithmetical Operations, and Calculators ...
SyllAbuS - Cambridge International Examinations
... A Level or from A Level to AS Level) are listed on pages 95 and 96. • Data Booklet: The Data Booklet for use with Papers 1, 2 and 4 has been updated. This syllabus is for examination in 2016, 2017 and 2018. If candidates have studied the 2015 syllabus please be aware of the following: • Assessment ...
... A Level or from A Level to AS Level) are listed on pages 95 and 96. • Data Booklet: The Data Booklet for use with Papers 1, 2 and 4 has been updated. This syllabus is for examination in 2016, 2017 and 2018. If candidates have studied the 2015 syllabus please be aware of the following: • Assessment ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... ◦ when changing the coefficient of a compound, the amounts of all the atoms are changed. ◦ do a recount of all the atoms every time you change a coefficient. ◦ do a final check at the end. ◦ fractions are not allowed. Multiply to reach whole numbers. ...
... ◦ when changing the coefficient of a compound, the amounts of all the atoms are changed. ◦ do a recount of all the atoms every time you change a coefficient. ◦ do a final check at the end. ◦ fractions are not allowed. Multiply to reach whole numbers. ...
Chemistry Science Notebook: Student Edition
... vocabulary, and develop their thinking skills by writing in order to achieve academic success. The ability to take and organize notes predicts how well students will do in school. Peverly, Brobst, Graham, and Shaw (2003) showed that when students use background knowledge and take notes, they are lik ...
... vocabulary, and develop their thinking skills by writing in order to achieve academic success. The ability to take and organize notes predicts how well students will do in school. Peverly, Brobst, Graham, and Shaw (2003) showed that when students use background knowledge and take notes, they are lik ...
SCH4U TEXT BOOK
... s you wander through the supermarket, some advertising claims catch your eye. “Certified organic” and “all natural” are stamped on the labels of some foods. Other labels claim that the foods are “chemical free.” As a chemistry student, you are aware that these labels may be misleading. Are all “chem ...
... s you wander through the supermarket, some advertising claims catch your eye. “Certified organic” and “all natural” are stamped on the labels of some foods. Other labels claim that the foods are “chemical free.” As a chemistry student, you are aware that these labels may be misleading. Are all “chem ...
synthesis and properties of v3+ analogues of jarosite-group
... thermal decomposition of the respective hydrated double sulfate salts under vacuum. However, the procedure requires close control of the temperature and appears to be applicable only for species having a volatile M ion, such as H3O or NH4. Dobley et al. (2000) synthesized the V3+ analogue of natroja ...
... thermal decomposition of the respective hydrated double sulfate salts under vacuum. However, the procedure requires close control of the temperature and appears to be applicable only for species having a volatile M ion, such as H3O or NH4. Dobley et al. (2000) synthesized the V3+ analogue of natroja ...
Calculations with Chemical Formulas and Equations
... The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
... The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... of the lower figure (240 nm) were collected in 1 second and the second fifty collected in 119 seconds. The family of traces results from stopped-flow experiments performed on the same solution at various times over a 2 hr period (t = 0 hr (A), t = 2 hr (B)) ...
... of the lower figure (240 nm) were collected in 1 second and the second fifty collected in 119 seconds. The family of traces results from stopped-flow experiments performed on the same solution at various times over a 2 hr period (t = 0 hr (A), t = 2 hr (B)) ...
Chapter 3 Stoichiometry: Calculations with Chemical
... The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
... The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...