Chapter 4: Reactions in Aqueous Solution
... 1) Water is a very common solvent due to its wide availability and low cost (most of our world is water). 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with wat ...
... 1) Water is a very common solvent due to its wide availability and low cost (most of our world is water). 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with wat ...
13.0 Redox Reactions PowerPoint
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
L-12 Spontaneity of chemical reactions
... the first law does not deny the possibility that a metal bar having a uniform temperature can spontaneously become warmer at one end and cooler at the other. But it is known from experience that such a change does not occur without expenditure of energy from an external source. The first law also st ...
... the first law does not deny the possibility that a metal bar having a uniform temperature can spontaneously become warmer at one end and cooler at the other. But it is known from experience that such a change does not occur without expenditure of energy from an external source. The first law also st ...
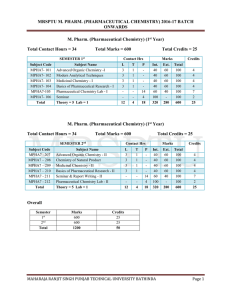
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Infrared Spectroscopy: The Hook’s Law and Calculation of Stretching Frequencies for Different Types of Bonds And Their Bond Strengths, Coupled Interactions, Hydrogen Bonding, Examination of Infrared Spectrum, Survey of Important Functional Groups With Examples, Radiation Source, Detectors Used, Samp ...
... Infrared Spectroscopy: The Hook’s Law and Calculation of Stretching Frequencies for Different Types of Bonds And Their Bond Strengths, Coupled Interactions, Hydrogen Bonding, Examination of Infrared Spectrum, Survey of Important Functional Groups With Examples, Radiation Source, Detectors Used, Samp ...
Unit 14-Chemical Reactions
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
+ H 2 O
... The Water Molecule bent shape and ability to hydrogen bond gives it many special properties. Water molecules are attracted to one another by dipole interactions This hydrogen bonding gives water: a) its high surface tension, b) its Water’s ...
... The Water Molecule bent shape and ability to hydrogen bond gives it many special properties. Water molecules are attracted to one another by dipole interactions This hydrogen bonding gives water: a) its high surface tension, b) its Water’s ...
2011-2012 Paper 1
... 6. Chlorine has a relative atomic mass of 35.5 and has two isotopes with relative isotopic masses of 35 and 37. Which of the following statements about chlorine are CORRECT? (1) The isotopes have same atomic number. (2) It contains the two isotopes, chlorine-35 and chlorine-37, in a ratio of 1:3. (3 ...
... 6. Chlorine has a relative atomic mass of 35.5 and has two isotopes with relative isotopic masses of 35 and 37. Which of the following statements about chlorine are CORRECT? (1) The isotopes have same atomic number. (2) It contains the two isotopes, chlorine-35 and chlorine-37, in a ratio of 1:3. (3 ...
Chapter 4
... 3) The oxidation state of oxygen in compounds is -2, except in peroxides, such as H2O2 where it is -1. 4) The oxidation state of hydrogen in compounds is +1, except in metal hydrides, like NaH, where it is -1. ...
... 3) The oxidation state of oxygen in compounds is -2, except in peroxides, such as H2O2 where it is -1. 4) The oxidation state of hydrogen in compounds is +1, except in metal hydrides, like NaH, where it is -1. ...
chapter 6 - thermochemistry
... Energy is defined as the capacity to do work or to produce heat. This chapter focuses specifically on the production or absorption of heat that accompanies chemical reactions. The law of the conservation of energy states that energy cannot be created nor destroyed, but it may be converted from one f ...
... Energy is defined as the capacity to do work or to produce heat. This chapter focuses specifically on the production or absorption of heat that accompanies chemical reactions. The law of the conservation of energy states that energy cannot be created nor destroyed, but it may be converted from one f ...
KINETICS questions
... (a) According to the data shown, what is the rate law for the reaction above? (b) On the basis of the rate law determined in part (a), calculate the specific rate constant. Specify the units. (c) What is the numerical value for the initial rate of disappearance of C2O42- for Experiment 1? (d) Calcul ...
... (a) According to the data shown, what is the rate law for the reaction above? (b) On the basis of the rate law determined in part (a), calculate the specific rate constant. Specify the units. (c) What is the numerical value for the initial rate of disappearance of C2O42- for Experiment 1? (d) Calcul ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.