AP Semester I Review: Free Response Questions
... water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4 (aq). The balanced equation for the reaction that occurred is as follows: 16 H+ (aq) + 2 MnO4- (aq) + 5 C2O42- (aq) 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) The volume of 0.0150 M KMnO4 (aq) required to rea ...
... water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4 (aq). The balanced equation for the reaction that occurred is as follows: 16 H+ (aq) + 2 MnO4- (aq) + 5 C2O42- (aq) 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) The volume of 0.0150 M KMnO4 (aq) required to rea ...
1 - kurtniedenzu
... difference in atomic radius. d. Each kind of atom has a characteristic difference in energy levels for electrons 19. Which property is most closely related to similarity in chemical properties of the elements? a. number of neutrons b. number of protons c. number of valence electrons d. number of occ ...
... difference in atomic radius. d. Each kind of atom has a characteristic difference in energy levels for electrons 19. Which property is most closely related to similarity in chemical properties of the elements? a. number of neutrons b. number of protons c. number of valence electrons d. number of occ ...
Chemistry Final Exam Study Guide_S2014
... 11. Draw an orbital diagram, complete electron configuration and noble gas notation for: a. Na b. C c. Mo d. Se 12. How does an electron become excited? What does it do when it returns to the ground state? 13. What is a photon? Quantum? 14. Describe the relationship between wavelength and frequency. ...
... 11. Draw an orbital diagram, complete electron configuration and noble gas notation for: a. Na b. C c. Mo d. Se 12. How does an electron become excited? What does it do when it returns to the ground state? 13. What is a photon? Quantum? 14. Describe the relationship between wavelength and frequency. ...
Miss Pang`s 2012 Review
... 19. In 1803, John Dalton, in an attempt to explain the findings of his work on the solubility of gases as well as the laws of Lav oiser and Proust, wrote the first atomic theory based on experimental fact. Which of the following statements does not correspond with Dalton’s theory? A) B) C) D) ...
... 19. In 1803, John Dalton, in an attempt to explain the findings of his work on the solubility of gases as well as the laws of Lav oiser and Proust, wrote the first atomic theory based on experimental fact. Which of the following statements does not correspond with Dalton’s theory? A) B) C) D) ...
File
... The positive charges of the protons are cancelled by the negative charges of the electrons, so overall an atom has a neutral charge. 6. The mass of a proton is 1 amu. The mass of a neutron is 1 amu. The mass of an electron is almost 0 amu. The amu is defined as 1/12 the mass of a Carbon atom. ...
... The positive charges of the protons are cancelled by the negative charges of the electrons, so overall an atom has a neutral charge. 6. The mass of a proton is 1 amu. The mass of a neutron is 1 amu. The mass of an electron is almost 0 amu. The amu is defined as 1/12 the mass of a Carbon atom. ...
What You Need To Know for the Chemistry Regents
... The positive charges of the protons are cancelled by the negative charges of the electrons, so overall an atom has a neutral charge. 6. The mass of a proton is 1 amu. The mass of a neutron is 1 amu. The mass of an electron is almost 0 amu. The amu is defined as 1/12 the mass of a Carbon atom. ...
... The positive charges of the protons are cancelled by the negative charges of the electrons, so overall an atom has a neutral charge. 6. The mass of a proton is 1 amu. The mass of a neutron is 1 amu. The mass of an electron is almost 0 amu. The amu is defined as 1/12 the mass of a Carbon atom. ...
the atomic theory
... 5. Neils Bohr 6. nucleus 7. proton 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
... 5. Neils Bohr 6. nucleus 7. proton 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
What You Need To Know for the Chemistry Regents Exam
... Electrons in Lewis structures are arranged by their orbitals. The first two electrons are placed together in the “s” orbital. The remaining electrons are spread among the 3 “p” orbitals. The “s” orbital must be filled first. Then each “p” orbital must have one electron before another “p” orb ...
... Electrons in Lewis structures are arranged by their orbitals. The first two electrons are placed together in the “s” orbital. The remaining electrons are spread among the 3 “p” orbitals. The “s” orbital must be filled first. Then each “p” orbital must have one electron before another “p” orb ...
Need
... Electrons in Lewis structures are arranged by their orbitals. The first two electrons are placed together in the “s” orbital. The remaining electrons are spread among the 3 “p” orbitals. The “s” orbital must be filled first. Then each “p” orbital must have one electron before another “p” orb ...
... Electrons in Lewis structures are arranged by their orbitals. The first two electrons are placed together in the “s” orbital. The remaining electrons are spread among the 3 “p” orbitals. The “s” orbital must be filled first. Then each “p” orbital must have one electron before another “p” orb ...
Review Chemistry KEY - cms16-17
... 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii. Atoms: C=6, H=8, and O=6 Total number of atoms=20___________ b. C8H10O2N4H2O (Caffe ...
... 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii. Atoms: C=6, H=8, and O=6 Total number of atoms=20___________ b. C8H10O2N4H2O (Caffe ...
Teacher quality grant - Gulf Coast State College
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
Teacher quality grant
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
ViewpointAPBiology
... Effect of electrons – chemical behavior of an atom depends on number of electrons in its outermost shell ...
... Effect of electrons – chemical behavior of an atom depends on number of electrons in its outermost shell ...
Hydrogen Bonding
... It is the cardinal rule of bonding. It is the gain in stability when atoms have a full complement of eight electrons in their valence shells. The bonding in carbon dioxide (CO2): all atoms are surrounded by 8 electrons, fulfilling the octet rule http://en.wikipedia.org/wiki/Octet_rule ...
... It is the cardinal rule of bonding. It is the gain in stability when atoms have a full complement of eight electrons in their valence shells. The bonding in carbon dioxide (CO2): all atoms are surrounded by 8 electrons, fulfilling the octet rule http://en.wikipedia.org/wiki/Octet_rule ...
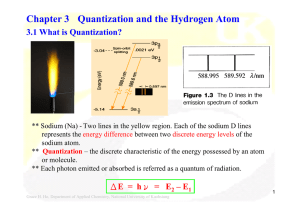
Chapter 3 Quantization and the Hydrogen Atom
... according to the aufbau principle. ** B2, C2, N2: σg2s and σg2p MO have the same symmetry, they push each other apart, thereby losing the symmetetrical disposition of the bonding and antibonding energy levels. Can explain the paramagnetic property of B2. ** O2 and F2: The pushing apart of σg2s and ...
... according to the aufbau principle. ** B2, C2, N2: σg2s and σg2p MO have the same symmetry, they push each other apart, thereby losing the symmetetrical disposition of the bonding and antibonding energy levels. Can explain the paramagnetic property of B2. ** O2 and F2: The pushing apart of σg2s and ...
Molecules
... diatomic molecule obey the selection rule Δ l = ± 1 and fall into two sequences: those for which Δ l = + 1 and those for which Δ l = - 1. The transition energies are given by Equation 11.14. (b) Expected lines in the optical absorption spectrum of a molecule. The lines on the right side of center co ...
... diatomic molecule obey the selection rule Δ l = ± 1 and fall into two sequences: those for which Δ l = + 1 and those for which Δ l = - 1. The transition energies are given by Equation 11.14. (b) Expected lines in the optical absorption spectrum of a molecule. The lines on the right side of center co ...
V. Chemical reactions
... e. How is the number of protons determined? by atomic number f. How is the number of neutrons determined? subtract atomic number from mass number g. How is the number of electrons determined in a neutral atom? Equal to the number of protons B) The nucleus a. What subatomic particles are located in t ...
... e. How is the number of protons determined? by atomic number f. How is the number of neutrons determined? subtract atomic number from mass number g. How is the number of electrons determined in a neutral atom? Equal to the number of protons B) The nucleus a. What subatomic particles are located in t ...
Chemistry Midterm Review 2006
... 3. Identify which ones have dipole-dipole forces? PBr3, N2, CF4, HBr, H2O 4. Identify which ones have London dispersion forces? , N2, CF4, HBr, SO2 5. Identify which ones have hydrogen bonding? HCl,, H2, HBr, H2O, CH4 6. Define the physical properties of Viscosity, Surface Tension, Boiling Point and ...
... 3. Identify which ones have dipole-dipole forces? PBr3, N2, CF4, HBr, H2O 4. Identify which ones have London dispersion forces? , N2, CF4, HBr, SO2 5. Identify which ones have hydrogen bonding? HCl,, H2, HBr, H2O, CH4 6. Define the physical properties of Viscosity, Surface Tension, Boiling Point and ...
A`r ji r/ Ii
... Alternative to (s); used only for a precipitate (solid) falling out of solution A reactant or product in the liquid state A reactant or product in aqueous solution (dissolved in water) A reactant or product in the gaseous state ...
... Alternative to (s); used only for a precipitate (solid) falling out of solution A reactant or product in the liquid state A reactant or product in aqueous solution (dissolved in water) A reactant or product in the gaseous state ...
Test Review: Unit 1 - Ms. Hill`s Pre
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
2. Essential Chemistry
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.