Main Group Notes 1
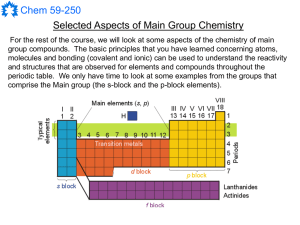
... For the rest of the course, we will look at some aspects of the chemistry of main group compounds. The basic principles that you have learned concerning atoms, molecules and bonding (covalent and ionic) can be used to understand the reactivity and structures that are observed for elements and compou ...
... For the rest of the course, we will look at some aspects of the chemistry of main group compounds. The basic principles that you have learned concerning atoms, molecules and bonding (covalent and ionic) can be used to understand the reactivity and structures that are observed for elements and compou ...
Bohr, Niels Henrik David
... their atoms and that only the atomic weight and possible radioactive behaviour are determined by the small but massive nucleus itself. Rutherford's nuclear atom was both mechanically and electromagnetically unstable, but Bohr imposed stability on it by introducing the new and not yet clarified idea ...
... their atoms and that only the atomic weight and possible radioactive behaviour are determined by the small but massive nucleus itself. Rutherford's nuclear atom was both mechanically and electromagnetically unstable, but Bohr imposed stability on it by introducing the new and not yet clarified idea ...
Chemistry 11th
... This is a permanent effect and is generally represented by an arrow with is head in the middle of the covalent bond pointing in the direction of displacement of electron as shown below : ...
... This is a permanent effect and is generally represented by an arrow with is head in the middle of the covalent bond pointing in the direction of displacement of electron as shown below : ...
File
... occupied by the SO2 molecules is A) the larger SO2 molecules cannot be treated as point sources, and occupy a significant portion of the container. B) the larger SO2 molecules move slower than the other gas particles, and therefore exert a smaller pressure on the container walls. C) there are signif ...
... occupied by the SO2 molecules is A) the larger SO2 molecules cannot be treated as point sources, and occupy a significant portion of the container. B) the larger SO2 molecules move slower than the other gas particles, and therefore exert a smaller pressure on the container walls. C) there are signif ...
3.091 Summary Lecture Notes, Fall 2009
... o ao= Bohr radius = 0.529 Angstroms E=0 o Z = proton number E3 =-KZ2/9 o e = elementary charge ...
... o ao= Bohr radius = 0.529 Angstroms E=0 o Z = proton number E3 =-KZ2/9 o e = elementary charge ...
N5 Chemistry Summary notes 2017
... Covalent bonds hold the atoms in a molecule together. Take Hydrogen (H2) as an example. Each hydrogen atom has 1 positive proton in the nucleus and 1 negative electron in its electron shell. Two hydrogen atoms share their outer electron to obtain a full outer shell. ...
... Covalent bonds hold the atoms in a molecule together. Take Hydrogen (H2) as an example. Each hydrogen atom has 1 positive proton in the nucleus and 1 negative electron in its electron shell. Two hydrogen atoms share their outer electron to obtain a full outer shell. ...
Student Expectation
... number of protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron ...
... number of protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron ...
Unit 10: Structure and Bonding
... Radioactive and Non radioactive isotopes Do NOT assume the word isotope means the atom it is radioactive, this depends on the stability of the nucleus i.e. unstable atoms (radioactive) might be referred to as radioisotopes. Many isotopes are extremely stable in the nuclear sense and NOT radioactive ...
... Radioactive and Non radioactive isotopes Do NOT assume the word isotope means the atom it is radioactive, this depends on the stability of the nucleus i.e. unstable atoms (radioactive) might be referred to as radioisotopes. Many isotopes are extremely stable in the nuclear sense and NOT radioactive ...
Which notation represents an atom of sodium
... The atom absorbs energy, and one or more electrons move to a higher electron shell. The atom absorbs energy, and one or more electrons move to a lower electron shell. The atom releases energy, and one or more electrons move to a higher electron shell. The atom releases energy, and one or more electr ...
... The atom absorbs energy, and one or more electrons move to a higher electron shell. The atom absorbs energy, and one or more electrons move to a lower electron shell. The atom releases energy, and one or more electrons move to a higher electron shell. The atom releases energy, and one or more electr ...
Compounds
... 1. How many sulfur atoms are in 0.25 moles of Sulfur? 2. How many moles of boron are in 3.79x1024 atoms of Boron? 3. What is the molar mass of N2O5? 4. How many grams are in 1.34 moles of Aspirin (C9H8O4)? 5. How many grams are in a 10 L tank of Propane (C3H8) at STP? 6. How many atoms of sulfur are ...
... 1. How many sulfur atoms are in 0.25 moles of Sulfur? 2. How many moles of boron are in 3.79x1024 atoms of Boron? 3. What is the molar mass of N2O5? 4. How many grams are in 1.34 moles of Aspirin (C9H8O4)? 5. How many grams are in a 10 L tank of Propane (C3H8) at STP? 6. How many atoms of sulfur are ...
O - gearju.com
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
O - gearju.com
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
Final Exam Review
... A) magnesium (Mg) C) sulfur (S) B) silicon (Si) D) argon (Ar) 19. Which of the following statements about the quantum-mechanical model for the atom is not true? (Ch. 11) a. Electrons exist only in shells which are a specific distance from the nucleus, determined by the principal quantum number, n. b ...
... A) magnesium (Mg) C) sulfur (S) B) silicon (Si) D) argon (Ar) 19. Which of the following statements about the quantum-mechanical model for the atom is not true? (Ch. 11) a. Electrons exist only in shells which are a specific distance from the nucleus, determined by the principal quantum number, n. b ...
SAT - mvhs-fuhsd.org
... Electron-Dot Diagrams • The number of dots equals the number of valence electrons. • The number of unpaired valence electrons in a nonmetal tells you how many covalent bonds that atom can form with other nonmetals or how many electrons it wants to gain from metals to form an ion. • The number of va ...
... Electron-Dot Diagrams • The number of dots equals the number of valence electrons. • The number of unpaired valence electrons in a nonmetal tells you how many covalent bonds that atom can form with other nonmetals or how many electrons it wants to gain from metals to form an ion. • The number of va ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.