On the Importance of Prereactive Complexes in
... CH3CH ) O + OH f [CH2CH ) O] + H2O ∆H298 -25 ( 2 (5) The behavior of reactions having a negative temperature dependence has been successfully described, for systems at low pressures, by Mozurkewich and Benson,10 and for systems at high pressures, by Singleton and Cvetanovic.11 In this case, several ...
... CH3CH ) O + OH f [CH2CH ) O] + H2O ∆H298 -25 ( 2 (5) The behavior of reactions having a negative temperature dependence has been successfully described, for systems at low pressures, by Mozurkewich and Benson,10 and for systems at high pressures, by Singleton and Cvetanovic.11 In this case, several ...
Chapter 18 review
... a. It is exothermic. b. It takes place at a rapid rate. c. It results in increased disorder of the system. d. It releases free energy. ____ 19. Which of the following is true about the combustion of carbon? a. The reaction is spontaneous. b. The reaction is endothermic. c. Enthalpy remains constant. ...
... a. It is exothermic. b. It takes place at a rapid rate. c. It results in increased disorder of the system. d. It releases free energy. ____ 19. Which of the following is true about the combustion of carbon? a. The reaction is spontaneous. b. The reaction is endothermic. c. Enthalpy remains constant. ...
Chemistry - CBSE Academic
... The new and updated curriculum is based on disciplinary approach with rigour and depth taking care that the syllabus is not heavy and at the same time it is comparable to the international level. The knowledge related to the subject of Chemistry has undergone tremendous changes during the past one d ...
... The new and updated curriculum is based on disciplinary approach with rigour and depth taking care that the syllabus is not heavy and at the same time it is comparable to the international level. The knowledge related to the subject of Chemistry has undergone tremendous changes during the past one d ...
9701/04 - StudyGuide.PK
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... 19. The molarity of a solution prepared by diluting 43.72 mL of 5.005 M aqueous K2Cr2O7 to 500 mL is __________. (a). 57.2 (b). 0.0044 (c). 0.438 (d). 0.0879 Explanation: You should use the M1V1 = M2V2 formula here. It is the only formula that you should use here since this is just a dilution of the ...
... 19. The molarity of a solution prepared by diluting 43.72 mL of 5.005 M aqueous K2Cr2O7 to 500 mL is __________. (a). 57.2 (b). 0.0044 (c). 0.438 (d). 0.0879 Explanation: You should use the M1V1 = M2V2 formula here. It is the only formula that you should use here since this is just a dilution of the ...
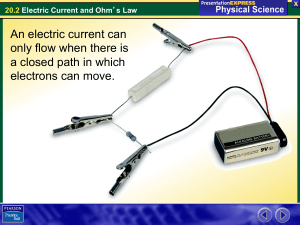
20.2 Electric Current and Ohm
... A metal is made up of ions in a lattice. The ions are not free to move. • Each ion has one or more electrons that are not tightly bound to it. • These free electrons can conduct charge. • Most materials do not easily conduct charge because they don’t have free electrons. ...
... A metal is made up of ions in a lattice. The ions are not free to move. • Each ion has one or more electrons that are not tightly bound to it. • These free electrons can conduct charge. • Most materials do not easily conduct charge because they don’t have free electrons. ...
PREPARATORY PROBLEMS (Theoretical)
... Nanochemistry has sparked much excitement in the recent years and a large amount of research has been dedicated to understanding of nanomaterials. Single-walled carbon nanotubes (SWNTs) are a universally known example of such materials. SWNT can be thought of as a sheet of graphite rolled into a sea ...
... Nanochemistry has sparked much excitement in the recent years and a large amount of research has been dedicated to understanding of nanomaterials. Single-walled carbon nanotubes (SWNTs) are a universally known example of such materials. SWNT can be thought of as a sheet of graphite rolled into a sea ...
PREPARATORY PROBLEMS (Theoretical)
... Nanochemistry has sparked much excitement in the recent years and a large amount of research has been dedicated to understanding of nanomaterials. Single-walled carbon nanotubes (SWNTs) are a universally known example of such materials. SWNT can be thought of as a sheet of graphite rolled into a sea ...
... Nanochemistry has sparked much excitement in the recent years and a large amount of research has been dedicated to understanding of nanomaterials. Single-walled carbon nanotubes (SWNTs) are a universally known example of such materials. SWNT can be thought of as a sheet of graphite rolled into a sea ...
Chapter 2 Geochemical Reactions
... A range of aqueous reactions involve the dissociation of ionic bonds and the formation and exchange of ions in solution. While often classified as acid-base reactions, they are more than this, ranging from simple acid dissociation reactions to mineral dissolution, ion hydration and formation of comp ...
... A range of aqueous reactions involve the dissociation of ionic bonds and the formation and exchange of ions in solution. While often classified as acid-base reactions, they are more than this, ranging from simple acid dissociation reactions to mineral dissolution, ion hydration and formation of comp ...
Document
... Skipped these in 2011-2012 Questions – 1. Nitrogen gas is also present in the atmosphere and it reacted with the Mg to magnesium nitride. Write a balanced chemical equation for this reaction. 2. When you added water to the crucible. The water reacts with the magnesium nitride (as heat is applied) t ...
... Skipped these in 2011-2012 Questions – 1. Nitrogen gas is also present in the atmosphere and it reacted with the Mg to magnesium nitride. Write a balanced chemical equation for this reaction. 2. When you added water to the crucible. The water reacts with the magnesium nitride (as heat is applied) t ...
Document
... Convert the mass of the Mg into moles. Convert the mass of the oxygen into moles. Are there more moles of Mg or oxygen? By how many times? Round to the nearest whole number. 7. What is the chemical formula for magnesium oxide? Explain how you know. ...
... Convert the mass of the Mg into moles. Convert the mass of the oxygen into moles. Are there more moles of Mg or oxygen? By how many times? Round to the nearest whole number. 7. What is the chemical formula for magnesium oxide? Explain how you know. ...
File
... Limiting Reactant • In the real world, reactants are not present in the exact mole ratio described by the balanced equation. • This means that one of the reactants will be used up before the other one. – The limiting reactant is used up first and restricts (stops) the reaction – The excess reactant ...
... Limiting Reactant • In the real world, reactants are not present in the exact mole ratio described by the balanced equation. • This means that one of the reactants will be used up before the other one. – The limiting reactant is used up first and restricts (stops) the reaction – The excess reactant ...
James Ruse with Solutions
... A soft drink may be decarbonated by heating. In observing the results, the equilibrium between gaseous and dissolved carbon dioxide can be examined. CO2 (g) ...
... A soft drink may be decarbonated by heating. In observing the results, the equilibrium between gaseous and dissolved carbon dioxide can be examined. CO2 (g) ...
Cheat Sheet for Chemical Equilibrium
... • The equilibrium constant for these problems is called the solubility product while the solubility is the concentration that is actually dissolved or “x”. • The way to show the dissolving of the solid is AgI Ag+ + Cl‐ • Since the reactants are solids, the equilibrium expression would be Ksp= [p ...
... • The equilibrium constant for these problems is called the solubility product while the solubility is the concentration that is actually dissolved or “x”. • The way to show the dissolving of the solid is AgI Ag+ + Cl‐ • Since the reactants are solids, the equilibrium expression would be Ksp= [p ...
N5 Chemistry 2014
... body requires a regular intake of potassium because humans have no mechanism for storing it. Foods rich in potassium include raisins and almonds. Raisins contain 0·86 g of potassium in every 100 g. Naturally occurring salts of potassium such as saltpetre (potassium nitrate) and potash (potassium car ...
... body requires a regular intake of potassium because humans have no mechanism for storing it. Foods rich in potassium include raisins and almonds. Raisins contain 0·86 g of potassium in every 100 g. Naturally occurring salts of potassium such as saltpetre (potassium nitrate) and potash (potassium car ...
program
... substance with an atomic lattice, or a metal: • atom bond or covalent bond; • polar bond; • hydrogen bond; • van der Waals bond; • ion bond; • metallic bond; • dipole-dipole bond. ...
... substance with an atomic lattice, or a metal: • atom bond or covalent bond; • polar bond; • hydrogen bond; • van der Waals bond; • ion bond; • metallic bond; • dipole-dipole bond. ...
13. Condensed azines. Quinoline. Isoquinoline. Acridine. Diazines
... with the molecular formulaC4H4N2, sometimes called 1,2-diazine. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. Pyridazine has no household use. It is mainly used in research and industry as building block for more complex co ...
... with the molecular formulaC4H4N2, sometimes called 1,2-diazine. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. Pyridazine has no household use. It is mainly used in research and industry as building block for more complex co ...
Chapter 4: Chemical Reaction Dynamics
... chemical reaction. Even in the absence of exact QM reactive-scattering calculations, important insight into chemical dynamics can be gained from analysing classical collision trajectories on the BO-PES. Consider the simplest polyatomic case: the reaction between an atom A and a diatom BC: A + BC → A ...
... chemical reaction. Even in the absence of exact QM reactive-scattering calculations, important insight into chemical dynamics can be gained from analysing classical collision trajectories on the BO-PES. Consider the simplest polyatomic case: the reaction between an atom A and a diatom BC: A + BC → A ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.