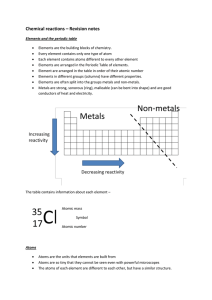
Chemical reactions revision
... You should be able to give the name of the compound formed when different elements combine and tell which elements are present in any simple compound ...
... You should be able to give the name of the compound formed when different elements combine and tell which elements are present in any simple compound ...
List of Definitions for AS Chemistry
... and some are absolutely specific, operating for only one substance in only one reaction. For reactions that normally produce a pair of optical isomers (racemic mixture) when carried out in the lab, enzymes are able to selectively produce one optical isomer in the body. A transition element is a d-bl ...
... and some are absolutely specific, operating for only one substance in only one reaction. For reactions that normally produce a pair of optical isomers (racemic mixture) when carried out in the lab, enzymes are able to selectively produce one optical isomer in the body. A transition element is a d-bl ...
Redox Balancing Worksheet
... definition of redox reactions, then, involves the gain and loss of electrons rather than the gain and loss of oxygen. In the reaction below, for example, sodium metal (Na) reacts with chlorine gas (Cl 2 ) in such a way that sodium atoms lose one electron each to chlorine atoms: 2 Na + Cl 2 → 2 NaCl ...
... definition of redox reactions, then, involves the gain and loss of electrons rather than the gain and loss of oxygen. In the reaction below, for example, sodium metal (Na) reacts with chlorine gas (Cl 2 ) in such a way that sodium atoms lose one electron each to chlorine atoms: 2 Na + Cl 2 → 2 NaCl ...
3rd Quarter Test
... 9) Which of the following liquids has the weakest intermolecular forces of attraction between its molecules? a) Xe (l) b) Kr (l) c) Ne (l) d) He (l) 10) Which type of bond exists in a molecule of hydrogen iodide? a) a polar covalent bond with an electronegativity difference of zero b) a polar covale ...
... 9) Which of the following liquids has the weakest intermolecular forces of attraction between its molecules? a) Xe (l) b) Kr (l) c) Ne (l) d) He (l) 10) Which type of bond exists in a molecule of hydrogen iodide? a) a polar covalent bond with an electronegativity difference of zero b) a polar covale ...
Double Replacement Reactions
... the product side. (Do not change subscripts when balancing!) Example: Write the equation for hydrogen and oxygen reacting to form water. ...
... the product side. (Do not change subscripts when balancing!) Example: Write the equation for hydrogen and oxygen reacting to form water. ...
chapt02_lecture from text
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found • No orbital can contain more than two el ...
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found • No orbital can contain more than two el ...
Chemistry Standards Review
... Atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. The chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are ionic. Large molecules (polymers), such ...
... Atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. The chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are ionic. Large molecules (polymers), such ...
Balancing Chemical Equations
... Introduction: The equation H2 + O2 H2O is unbalanced because there are two oxygen atoms on the reactants side of the equation, and only one on the products side of the equation. To balance the equation, you cannot change the structure of any of the molecules, but you can change the number of molec ...
... Introduction: The equation H2 + O2 H2O is unbalanced because there are two oxygen atoms on the reactants side of the equation, and only one on the products side of the equation. To balance the equation, you cannot change the structure of any of the molecules, but you can change the number of molec ...
ppt
... The enthalpy of reaction is indicated by a separate expression beside the chemical equation »Remember: exothermic reactions have -ΔH endothermic reactions have +ΔH ...
... The enthalpy of reaction is indicated by a separate expression beside the chemical equation »Remember: exothermic reactions have -ΔH endothermic reactions have +ΔH ...
Miss Pang`s 2012 Review
... Each of these elements possesses the greatest atomic radius of its period. These six elements all possess the same electronic configuration. These six elements all possess only one electron in their outer energy level. These six elements all possess an odd number of electrons. ...
... Each of these elements possesses the greatest atomic radius of its period. These six elements all possess the same electronic configuration. These six elements all possess only one electron in their outer energy level. These six elements all possess an odd number of electrons. ...
Water: The Universal Solvent
... CaC2O4. The CaC2O4 was dissolved in sulfuric acid and the resulting H2C2O4 was titrated with a standard KMnO4 solution. (a) Write the balanced equation for the titration reaction, shown unbalanced ...
... CaC2O4. The CaC2O4 was dissolved in sulfuric acid and the resulting H2C2O4 was titrated with a standard KMnO4 solution. (a) Write the balanced equation for the titration reaction, shown unbalanced ...
Definitions - Loreto Science
... in a pure stable soluble solid form so that it can be weighed out and dissolved in water to give a solution of accurately known concentration. • (Know why high Mr matters) AG ...
... in a pure stable soluble solid form so that it can be weighed out and dissolved in water to give a solution of accurately known concentration. • (Know why high Mr matters) AG ...
Chemical Equations
... • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compounds. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction Types: Combustion •Combustion, at its mos ...
... • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compounds. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction Types: Combustion •Combustion, at its mos ...
Differentiated Chemistry First Term Test Review
... Sulfuric acid reacts with sodium hydroxide to yield sodium sulfate and water. If 37 grams of sulfuric acid react with 30. grams of sodium hydroxide, how many grams of water are produced? (A) 53 g (B) 14 g (C) 37 g (D) 45 g (E) 106 g ...
... Sulfuric acid reacts with sodium hydroxide to yield sodium sulfate and water. If 37 grams of sulfuric acid react with 30. grams of sodium hydroxide, how many grams of water are produced? (A) 53 g (B) 14 g (C) 37 g (D) 45 g (E) 106 g ...
普通化学 (全英文) 教学大纲
... How to find out the combination coefficients of different sub-reactions? 9.7.Standard States (XoT): For a pure solid or liquid they are standard states, concentration = 1 For a gas Standard states: Ppartial = 1 atm For an ion in solution Standard states: [ion] = 1 M If non-standard s ...
... How to find out the combination coefficients of different sub-reactions? 9.7.Standard States (XoT): For a pure solid or liquid they are standard states, concentration = 1 For a gas Standard states: Ppartial = 1 atm For an ion in solution Standard states: [ion] = 1 M If non-standard s ...
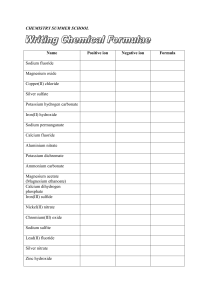
chemistry summer school
... Silver nitrate and copper(II) chloride solutions combine to form solid silver chloride and a solution of copper(II) nitrate. Sulfuric acid reacts with aluminium oxide powder. Glucose (C6H12O6) is fermented to carbon dioxide and ethanol (C2H5OH) Lead(II) nitrate and potassium iodide solutions combine ...
... Silver nitrate and copper(II) chloride solutions combine to form solid silver chloride and a solution of copper(II) nitrate. Sulfuric acid reacts with aluminium oxide powder. Glucose (C6H12O6) is fermented to carbon dioxide and ethanol (C2H5OH) Lead(II) nitrate and potassium iodide solutions combine ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.