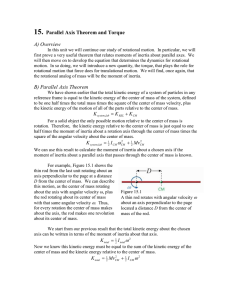
Document
... – Electrons are promoted through an electric discharge, heat, or some other source of energy – An atom in an excited state eventually emits photons as the electron drops back down to the ground state ...
... – Electrons are promoted through an electric discharge, heat, or some other source of energy – An atom in an excited state eventually emits photons as the electron drops back down to the ground state ...
Study Guide For Final File
... 7. Please explain the proper placement of the independent and dependent variable on a graph. (p.16) 8. The common unit of time most used by physicists and other scientists is the_________. (p.8) 9. The metric system is based on the power of this number_______. (p. 12) 10. The following metric prefix ...
... 7. Please explain the proper placement of the independent and dependent variable on a graph. (p.16) 8. The common unit of time most used by physicists and other scientists is the_________. (p.8) 9. The metric system is based on the power of this number_______. (p. 12) 10. The following metric prefix ...
File - USNA
... the total change in momentum of the collision, ΔpF + ΔpM, to be zero. The addition of Equations (2.40) and (2.44) clearly does not give zero. Linear momentum is not conserved if we use the conventions for momentum from classical physics even if we use the velocity transformation equations from the ...
... the total change in momentum of the collision, ΔpF + ΔpM, to be zero. The addition of Equations (2.40) and (2.44) clearly does not give zero. Linear momentum is not conserved if we use the conventions for momentum from classical physics even if we use the velocity transformation equations from the ...
Quantum Mechanics
... • The model correctly fits the quantized energy levels of the hydrogen atom and postulates only certain allowed circular orbits for the electron • Bohr’s model is incorrect. This model only works for hydrogen • Electrons do not move around the nucleus in circular orbits ...
... • The model correctly fits the quantized energy levels of the hydrogen atom and postulates only certain allowed circular orbits for the electron • Bohr’s model is incorrect. This model only works for hydrogen • Electrons do not move around the nucleus in circular orbits ...
MP350 Classical Mechanics Jon-Ivar Skullerud October 16, 2014
... Once we have found the equation of motion for θ, and the solution to this equation, we can go back and calculate x and z as functions of time. However, in the example of the simple pendulum, we are not usually interested in this. We note that the mass m does not appear in the equation of motion. We ...
... Once we have found the equation of motion for θ, and the solution to this equation, we can go back and calculate x and z as functions of time. However, in the example of the simple pendulum, we are not usually interested in this. We note that the mass m does not appear in the equation of motion. We ...