Hooke`s Law and Potential Energy
... and the force is positive and points downwards. The work done by the spring force over some small displacement x is given by W = Fxx. Recall that the work done by a conservative force, such as a spring force, is the difference between the initial value of the potential energy function and the fina ...
... and the force is positive and points downwards. The work done by the spring force over some small displacement x is given by W = Fxx. Recall that the work done by a conservative force, such as a spring force, is the difference between the initial value of the potential energy function and the fina ...
l - Westgate Mennonite Collegiate
... properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
... properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
2014-3 Human Power Notes
... The definition of a system is arbitrary. For example, we can think of all the blood in the body as a system. The heart needs to do mechanical work on it to increase its kinetic energy as it leaves the heart or else the blood kinetic energy will keep dissipating to heat and come to a stop. Other exam ...
... The definition of a system is arbitrary. For example, we can think of all the blood in the body as a system. The heart needs to do mechanical work on it to increase its kinetic energy as it leaves the heart or else the blood kinetic energy will keep dissipating to heat and come to a stop. Other exam ...
Document
... • Gravitational force: magnitude = mg, always vertical, pointing down. • sometimes does positive work, sometimes negative work. • Normal force: magnitude =whatever is needed for the objects not to penetrate surface; always perpendicular to surface. • never does any work !! • Friction force: ma ...
... • Gravitational force: magnitude = mg, always vertical, pointing down. • sometimes does positive work, sometimes negative work. • Normal force: magnitude =whatever is needed for the objects not to penetrate surface; always perpendicular to surface. • never does any work !! • Friction force: ma ...
Units of Energy Forms of Energy Goals for learning in
... • Temperature measures the average kinetic energy. So, if 10 particles had the same kinetic energy E1, then temperature would also be given by E1. • Heat is, on ther other hand, given by thermal energy, energy or the total kinetic energy of all particles. Therefore, heat is given by 10E1 as opposed ...
... • Temperature measures the average kinetic energy. So, if 10 particles had the same kinetic energy E1, then temperature would also be given by E1. • Heat is, on ther other hand, given by thermal energy, energy or the total kinetic energy of all particles. Therefore, heat is given by 10E1 as opposed ...
Problems for Mathematics of Motion: week 6
... the second test for this module. This test will take place on Monday 19 February. You do not need to hand in group solutions for these questions. Question 3 should be discussed in the groups, and handed in as usual before the class on Monday 19 February. 1 At time t seconds, a particle of mass m = 1 ...
... the second test for this module. This test will take place on Monday 19 February. You do not need to hand in group solutions for these questions. Question 3 should be discussed in the groups, and handed in as usual before the class on Monday 19 February. 1 At time t seconds, a particle of mass m = 1 ...
Characteristics of Waves
... There are 3 basic rules, named after the scientists that discovered them, that govern the filling of these orbitals with electrons… ...
... There are 3 basic rules, named after the scientists that discovered them, that govern the filling of these orbitals with electrons… ...
From the Big Bang to String Theory
... wound up where it is now by starting off somewhere in here: the `past’ is t < 0. ...
... wound up where it is now by starting off somewhere in here: the `past’ is t < 0. ...
Reaction Kinetics
... • Not all reactions go completely to completion (all reactants are used up) • Instead, some reactions can occur both forward and reverse at the same time. A reversible reaction is symbolized by double arrows • 2SO2(g) + O2(g) 2SO3(g) ...
... • Not all reactions go completely to completion (all reactants are used up) • Instead, some reactions can occur both forward and reverse at the same time. A reversible reaction is symbolized by double arrows • 2SO2(g) + O2(g) 2SO3(g) ...
Arnold’s Cat Map - Physics Department
... • Time evolution of the universe: deterministic chaos v. random behavior. • Sensitive dependence on initial conditions: Initially similar systems will move together, but will diverge as time evolves. • Ergodic: a system will return to its initial state after a given time. Underlying order to chaos? ...
... • Time evolution of the universe: deterministic chaos v. random behavior. • Sensitive dependence on initial conditions: Initially similar systems will move together, but will diverge as time evolves. • Ergodic: a system will return to its initial state after a given time. Underlying order to chaos? ...
8-1-potential energy - High Point University
... where m is the rest mass of the particle. It is the only kind of energy that a particle can have, although it is often convenient to write it as E = K + mc2 . The Energy Principle for a Particle says that the change in a particle’s energy is equal to the total work done on the particle. ∆E = W ...
... where m is the rest mass of the particle. It is the only kind of energy that a particle can have, although it is often convenient to write it as E = K + mc2 . The Energy Principle for a Particle says that the change in a particle’s energy is equal to the total work done on the particle. ∆E = W ...
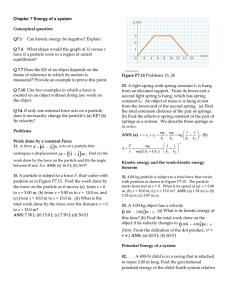
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
... as only Batman knows how, eventually getting it to swing enough that he can reach a ledge when the rope makes a 60.0° angle with the vertical. How much work was done by the gravitational force on Batman in this ...
... as only Batman knows how, eventually getting it to swing enough that he can reach a ledge when the rope makes a 60.0° angle with the vertical. How much work was done by the gravitational force on Batman in this ...
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
... Chapter 7 Energy of a system Conceptual question Q7.1 ...
... Chapter 7 Energy of a system Conceptual question Q7.1 ...
Unit 3 CPRx
... These are the Unit 3 skills needed to be mastered before the end of the first semester. You will be presented with opportunities to show your mastery (at least 3.0) of the Checkpoints (formative assessments) throughout this unit and till the end of the first semester. As we address each daily object ...
... These are the Unit 3 skills needed to be mastered before the end of the first semester. You will be presented with opportunities to show your mastery (at least 3.0) of the Checkpoints (formative assessments) throughout this unit and till the end of the first semester. As we address each daily object ...
Unit 4 Work, Energy and Power
... Listen to the next part of the text and number the paragraphs (including the equations) according to the correct order. Work and kinetic energy You can do work on an object to change its kinetic energy. In fact, the work done on a body is equal to its change in kinetic energy. (This is called the wo ...
... Listen to the next part of the text and number the paragraphs (including the equations) according to the correct order. Work and kinetic energy You can do work on an object to change its kinetic energy. In fact, the work done on a body is equal to its change in kinetic energy. (This is called the wo ...