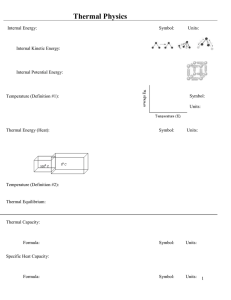
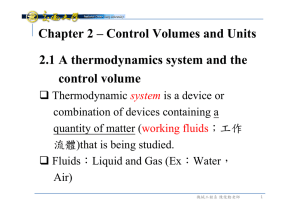
Document
... Mechanical Energy Conservation – Work Energy Theorem. ●When only Conservative forces act on an object (Fg, Fsp, Fn) the Mechanical Energy of that object must be conserved: ME = ME at any two points in the problem. Changes in KE or PE must simply transfer from one to another. The work done in the pro ...
... Mechanical Energy Conservation – Work Energy Theorem. ●When only Conservative forces act on an object (Fg, Fsp, Fn) the Mechanical Energy of that object must be conserved: ME = ME at any two points in the problem. Changes in KE or PE must simply transfer from one to another. The work done in the pro ...
Physical Chemistry: An Indian Journal
... Here the energy depends on two variables. The author of [2325] stated that the characterization of gas using Eq. (5) was a wrong approach because the physical quantity internal energy must depend on two variables (not on one). It must also depend on volume. He argued that equations of state with tw ...
... Here the energy depends on two variables. The author of [2325] stated that the characterization of gas using Eq. (5) was a wrong approach because the physical quantity internal energy must depend on two variables (not on one). It must also depend on volume. He argued that equations of state with tw ...
unit 9: thermal physics
... 1. Describe and explain the process of phase changes in terms of molecular behavior. When thermal energy is added to a solid, the molecules gain kinetic energy as they vibrate at an increased rate. This is seen macroscopically as an increase in temperature. At the melting point, a temperature is re ...
... 1. Describe and explain the process of phase changes in terms of molecular behavior. When thermal energy is added to a solid, the molecules gain kinetic energy as they vibrate at an increased rate. This is seen macroscopically as an increase in temperature. At the melting point, a temperature is re ...
Laws of Energy - SJSU Engineering
... meters away. How much Work does this person has to do to push the car to the gas station? Work = Force x Distance ...
... meters away. How much Work does this person has to do to push the car to the gas station? Work = Force x Distance ...
Laws_of_Energy_S12 - San Jose State University
... meters away. How much Work does this person has to do to push the car to the gas station? Work = Force x Distance ...
... meters away. How much Work does this person has to do to push the car to the gas station? Work = Force x Distance ...
Chem 11, Notes – Unit 3 – Properties of Matter
... What is the difference between a physical and chemical property? • A physical property of a pure substance is anything that can be observed without changing the identity of the substance o An intensive physical property depends upon the nature of the substance o An extensive physical property depend ...
... What is the difference between a physical and chemical property? • A physical property of a pure substance is anything that can be observed without changing the identity of the substance o An intensive physical property depends upon the nature of the substance o An extensive physical property depend ...
Stark shift of an on-center donor binding energy
... Disc-Shaped Quantum Dot in the Presence of Pressure and Temperature We have theoretically studied the combination effects of the electric and magnetic fields on the binding energy of an on-center donor impurity in disc-shaped GaAs/Al0.3Ga0.7As quantum dots (QDs) with emphasis on the competition effe ...
... Disc-Shaped Quantum Dot in the Presence of Pressure and Temperature We have theoretically studied the combination effects of the electric and magnetic fields on the binding energy of an on-center donor impurity in disc-shaped GaAs/Al0.3Ga0.7As quantum dots (QDs) with emphasis on the competition effe ...
Work and Energy
... You are traveling behind a truck on the highway. Knowing that you need to leave 100 ft when traveling at 30 mph to stop, you figure that you can leave 200 ft between you and the next car if you are going 60 mph…in case you need to suddenly stop. The truck in front of you suddenly stops to avoid an a ...
... You are traveling behind a truck on the highway. Knowing that you need to leave 100 ft when traveling at 30 mph to stop, you figure that you can leave 200 ft between you and the next car if you are going 60 mph…in case you need to suddenly stop. The truck in front of you suddenly stops to avoid an a ...
Homework #3: Conservation of Energy
... The first term under the quadratic is about 1000 times smaller than the second term, indicating that the problem could have been approximated by not even including gravitational PE for the final position. If that approximation would have been made, the result would have been found by taking the nega ...
... The first term under the quadratic is about 1000 times smaller than the second term, indicating that the problem could have been approximated by not even including gravitational PE for the final position. If that approximation would have been made, the result would have been found by taking the nega ...
REU 21st - Department of Physics and Astronomy
... Nature has revealed a beautiful secret! The behavior of the Universe becomes very simple if it is described in a way in which space and time are symmetric. ...
... Nature has revealed a beautiful secret! The behavior of the Universe becomes very simple if it is described in a way in which space and time are symmetric. ...
8.044 Lecture Notes Chapter 9: Quantum Ideal Gases
... Exclusion principle, which prevents us from putting more than one particle in each state. For N λV3 , the classical analysis is fine – we needn’t worry about the particles needing to th occupy the same 1-particle state. ...
... Exclusion principle, which prevents us from putting more than one particle in each state. For N λV3 , the classical analysis is fine – we needn’t worry about the particles needing to th occupy the same 1-particle state. ...
Paradigm - RHIP - UT Austin - The University of Texas at Austin
... fn(E)/fn’(E) given by statistics only. Difference between MCE and CE vanishes as the size of the system N increases. This type of “thermal” behavior requires no rescattering and no interactions. The collisions simply serve as a mechanism to populate phase space without ever reaching thermal or che ...
... fn(E)/fn’(E) given by statistics only. Difference between MCE and CE vanishes as the size of the system N increases. This type of “thermal” behavior requires no rescattering and no interactions. The collisions simply serve as a mechanism to populate phase space without ever reaching thermal or che ...
Week - Mat-Su School District
... Room 123 Description: This is an advanced Science course designed to prepare the student for either college Chemistry or AP Chemistry. The course covers the equivalent of one full year of general Chemistry, comparable to a first year course at a college or university. The course is a rigorous math-b ...
... Room 123 Description: This is an advanced Science course designed to prepare the student for either college Chemistry or AP Chemistry. The course covers the equivalent of one full year of general Chemistry, comparable to a first year course at a college or university. The course is a rigorous math-b ...
Solutions to MR6T: Conservation of Energy
... measured from the surface of the Earth. This is an arbitrary but convenient zero point, as all we can really measure is changes in energy anyway. b. If you define the surface of the Earth as the zero gravitational potential energy, then anywhere below the surface is negative, for example down a mine ...
... measured from the surface of the Earth. This is an arbitrary but convenient zero point, as all we can really measure is changes in energy anyway. b. If you define the surface of the Earth as the zero gravitational potential energy, then anywhere below the surface is negative, for example down a mine ...
Relativity 4 Relativistic Momentum
... As we have learned, mass is a form of potential energy. It can be converted into energy, or energy can be converted into mass. Because of this, mass does not have to be conserved in reactions. If you throw two balls at each other and they stick together (an inelastic collision), the resulting mass i ...
... As we have learned, mass is a form of potential energy. It can be converted into energy, or energy can be converted into mass. Because of this, mass does not have to be conserved in reactions. If you throw two balls at each other and they stick together (an inelastic collision), the resulting mass i ...
classical notions of heterogeneous freezing
... If a formed ice nucleus is too small (known as an unstable nucleus or "embryo"), the energy that would be released by forming its volume (negative change) is not enough to create its surface (positive change) then nucleation does not proceed. The formed nucleus should reach some critical size (or r ...
... If a formed ice nucleus is too small (known as an unstable nucleus or "embryo"), the energy that would be released by forming its volume (negative change) is not enough to create its surface (positive change) then nucleation does not proceed. The formed nucleus should reach some critical size (or r ...
What is energy?
... transferred is measured by how much work is done – energy and work are expressed in the same unit. But - energy can be present in an object or a system when nothing is happening. However – it can only be observed when it is transferred from one object or system to another. Measured in Joules ...
... transferred is measured by how much work is done – energy and work are expressed in the same unit. But - energy can be present in an object or a system when nothing is happening. However – it can only be observed when it is transferred from one object or system to another. Measured in Joules ...
Chp 19- reaction rates and equilibrium
... System—the part of the universe on which you focus your attention Surroundings—everything else in the universe Universe—the system and the surroundings Conservation of energy—states that in any chemical or physical process, energy is neither created nor destroyed; all can be accounted for as work, s ...
... System—the part of the universe on which you focus your attention Surroundings—everything else in the universe Universe—the system and the surroundings Conservation of energy—states that in any chemical or physical process, energy is neither created nor destroyed; all can be accounted for as work, s ...
Lecture 5: Spectroscopy and Photochemistry I
... • Infrared radiation (λ = 0.8 - 300 µm) – Excites vibrational motions in molecules – With a very few exceptions, infrared radiation is not energetic enough to break molecules or initiate photochemical processes ...
... • Infrared radiation (λ = 0.8 - 300 µm) – Excites vibrational motions in molecules – With a very few exceptions, infrared radiation is not energetic enough to break molecules or initiate photochemical processes ...
mr06Tsol
... measured from the surface of the Earth. This is an arbitrary but convenient zero point, as all we can really measure is changes in energy anyway. b. If you define the surface of the Earth as the zero gravitational potential energy, then anywhere below the surface is negative, for example down a mine ...
... measured from the surface of the Earth. This is an arbitrary but convenient zero point, as all we can really measure is changes in energy anyway. b. If you define the surface of the Earth as the zero gravitational potential energy, then anywhere below the surface is negative, for example down a mine ...