Test 3 Review
... is true. When a ball is tossed in the air it slows down until its speed reaches zero. This means the kinetic energy decreases to zero. But the speed reaches zero at he highest point where the potential energy is greatest. Energy is not being lost. It is just changing from kinetic energy to potential ...
... is true. When a ball is tossed in the air it slows down until its speed reaches zero. This means the kinetic energy decreases to zero. But the speed reaches zero at he highest point where the potential energy is greatest. Energy is not being lost. It is just changing from kinetic energy to potential ...
File
... (e) Flow of water from a hill to the ground. (f) Mixing of gases. In all the above cases, the system reaches a state of greater disorder. Eventhough the energy of the system increases (endothermic) during the above changes the processes are spontaneous because they are accompanied with increase in e ...
... (e) Flow of water from a hill to the ground. (f) Mixing of gases. In all the above cases, the system reaches a state of greater disorder. Eventhough the energy of the system increases (endothermic) during the above changes the processes are spontaneous because they are accompanied with increase in e ...
Unit 6: Work and Energy - myLearning | Pasco County Schools
... -The students will be placed into groups of 3-4. To them it will look random, but I will control the who gets placed in which group. -They will work at each station for 5 mins and answer the questions then rotate to another station. -The students can work as a cooperative group to answer each questi ...
... -The students will be placed into groups of 3-4. To them it will look random, but I will control the who gets placed in which group. -They will work at each station for 5 mins and answer the questions then rotate to another station. -The students can work as a cooperative group to answer each questi ...
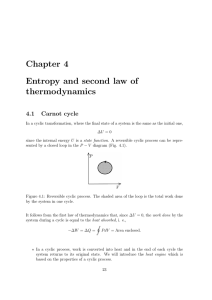
Chapter 4 Entropy and second law of thermodynamics
... (there is no law against this) (Fig. 4.5). Thus, Clausius’ Figure 4.5: Q2 = W = statement would be negated. Q1 process. (2) If Clausius’ statement were false, we could let an amount of heat Q1 flow from T2 to T1 (T2 < T1 ). Then, we could connect a Carnot engine between T1 and T2 such as to extract ...
... (there is no law against this) (Fig. 4.5). Thus, Clausius’ Figure 4.5: Q2 = W = statement would be negated. Q1 process. (2) If Clausius’ statement were false, we could let an amount of heat Q1 flow from T2 to T1 (T2 < T1 ). Then, we could connect a Carnot engine between T1 and T2 such as to extract ...
$doc.title
... Thermodynamics is the branch of physics devoted to the study of energy processes, which involve heat, mechanical work, and other aspects of energy and energy transfer, and the relationship between processes and thermal properties of matter. The unit begins by introducing the concept of temperature a ...
... Thermodynamics is the branch of physics devoted to the study of energy processes, which involve heat, mechanical work, and other aspects of energy and energy transfer, and the relationship between processes and thermal properties of matter. The unit begins by introducing the concept of temperature a ...
Convective heat transfer
... To quantify the ease with which a particular medium conducts, engineers employ the thermal conductivity, also known as the conductivity constant or conduction coefficient, k. In thermal conductivity, k is defined as "the quantity of heat, Q, transmitted in time (t) through a thickness (L), in a dir ...
... To quantify the ease with which a particular medium conducts, engineers employ the thermal conductivity, also known as the conductivity constant or conduction coefficient, k. In thermal conductivity, k is defined as "the quantity of heat, Q, transmitted in time (t) through a thickness (L), in a dir ...
P2 Knowledge Powerpoint
... • Kinetic Energy – movement energy • Gravitational Potential Energy – the energy something has due to its position relative to Earth – i.e. its height. Conservation of Energy When energy is transferred, the total amount always remains the ...
... • Kinetic Energy – movement energy • Gravitational Potential Energy – the energy something has due to its position relative to Earth – i.e. its height. Conservation of Energy When energy is transferred, the total amount always remains the ...
What is Energy? - Princeton High School
... Kinetic energy is energy in motion—the motion of electromagnetic waves, electrons, atoms, molecules, substances, and objects. Forms of kinetic energy include: Electrical energy is the movement of electrons. Everything is made of tiny particles called atoms. Atoms are made of even smaller particles ...
... Kinetic energy is energy in motion—the motion of electromagnetic waves, electrons, atoms, molecules, substances, and objects. Forms of kinetic energy include: Electrical energy is the movement of electrons. Everything is made of tiny particles called atoms. Atoms are made of even smaller particles ...
gravitational fields
... energy than one that sits at the ground floor of the building. – A compressed spring has energy stored in it. – Mass energy: Matter can be converted into energy (Einstein’s famous equation). ...
... energy than one that sits at the ground floor of the building. – A compressed spring has energy stored in it. – Mass energy: Matter can be converted into energy (Einstein’s famous equation). ...
Alignment to Michigan Educational Standards- Physical Science Safety
... systems, work is the amount of energy transferred as an object is moved through a distance, W = F d, where d is in the same direction as F. The total work done on an object depends on the net force acting on the object and the object’s displacement. Explain why work has a more precise scientific mea ...
... systems, work is the amount of energy transferred as an object is moved through a distance, W = F d, where d is in the same direction as F. The total work done on an object depends on the net force acting on the object and the object’s displacement. Explain why work has a more precise scientific mea ...
Chapter 2 PROPERTIES OF FLUIDS
... • Saturation pressure Psat: The pressure at which a pure substance changes phase at a given temperature. • Vapor pressure (Pv): The pressure exerted by its vapor in phase equilibrium with its liquid at a given temperature. It is identical to the saturation pressure Psat of the liquid (Pv = Psat). • ...
... • Saturation pressure Psat: The pressure at which a pure substance changes phase at a given temperature. • Vapor pressure (Pv): The pressure exerted by its vapor in phase equilibrium with its liquid at a given temperature. It is identical to the saturation pressure Psat of the liquid (Pv = Psat). • ...
Application of Thomas-Fermi model to a negative hydrogen ion in a
... There is a strong attractive center in atom, the nucleus, which acts on the electrons in an atom and to a large extent determines all the atomic properties. The electrons occupy given energy levels, forming shells and subshells. All this is understandable without accounting for the interelectron int ...
... There is a strong attractive center in atom, the nucleus, which acts on the electrons in an atom and to a large extent determines all the atomic properties. The electrons occupy given energy levels, forming shells and subshells. All this is understandable without accounting for the interelectron int ...
mapping fields
... motion tends to remain in that state of motion unless an external force is applied to it. ...
... motion tends to remain in that state of motion unless an external force is applied to it. ...