Physics (SPA)
... ideas of the motion of objects, for example the flawed idea that heavy objects fall faster than lighter ones, which dominated physics for about 2000 years. The greatest contribution to the development of mechanics is by one of the greatest physicists of all time, Isaac Newton. By extending Galileo’s ...
... ideas of the motion of objects, for example the flawed idea that heavy objects fall faster than lighter ones, which dominated physics for about 2000 years. The greatest contribution to the development of mechanics is by one of the greatest physicists of all time, Isaac Newton. By extending Galileo’s ...
midterm review for 2
... 1. A certain gas is compressed adiabatically. The amount of work done on the gas is 800 J. What is the change in the internal (thermal) energy of the gas? A) 800 J B) -800 J C) 400 J D) 0 J E) More information is needed to answer this question. 2. A cyclic process is carried out on an ideal gas such ...
... 1. A certain gas is compressed adiabatically. The amount of work done on the gas is 800 J. What is the change in the internal (thermal) energy of the gas? A) 800 J B) -800 J C) 400 J D) 0 J E) More information is needed to answer this question. 2. A cyclic process is carried out on an ideal gas such ...
Thermodynamics and Kinetics
... open system exchanges both, and an isolated system exchanges neither. • State function — the property of a system that depends only on the present state of the system and not on its history. ...
... open system exchanges both, and an isolated system exchanges neither. • State function — the property of a system that depends only on the present state of the system and not on its history. ...
Lec13drs
... Ad Ad 2Ad Inserting our formula for the capacitance of a parallel plate capacitor we find ...
... Ad Ad 2Ad Inserting our formula for the capacitance of a parallel plate capacitor we find ...
Chapter 4
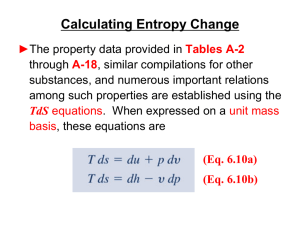
... (373.15 K). From Table A-2, (hg – hf) = 2257.1 kJ/kg. Thus (sg – sf) = (2257.1 kJ/kg)/373.15 K = 6.049 kJ/kg∙K which agrees with the value from Table A-2, as expected. ...
... (373.15 K). From Table A-2, (hg – hf) = 2257.1 kJ/kg. Thus (sg – sf) = (2257.1 kJ/kg)/373.15 K = 6.049 kJ/kg∙K which agrees with the value from Table A-2, as expected. ...
2016 Pre-University H2 Physics
... 1.1 Defining the systems under study (by specifying their boundaries and making explicit models of the systems) provides tools for understanding and testing ideas that are applicable throughout physics. 1.2 Objects can be treated as having no internal structure or an internal structure that can be i ...
... 1.1 Defining the systems under study (by specifying their boundaries and making explicit models of the systems) provides tools for understanding and testing ideas that are applicable throughout physics. 1.2 Objects can be treated as having no internal structure or an internal structure that can be i ...
Chapter 2 Motion Along a Straight Line Position, Displacement
... (a) How far apart would the equipotential surfaces be between the plates, if their potential difference was to be 0.10 V? Since the electric field is constant between the plates the equipotential surfaces will be evenly spaced (unlike ...
... (a) How far apart would the equipotential surfaces be between the plates, if their potential difference was to be 0.10 V? Since the electric field is constant between the plates the equipotential surfaces will be evenly spaced (unlike ...
Electric fields are
... Electric Potential Energy can be considered unrealized kinetic energy for a particle in a fixed location. It takes work to move a particle from a position of lower potential energy to a position of higher potential energy. This increase in potential energy at rest may be converted to (or “realized a ...
... Electric Potential Energy can be considered unrealized kinetic energy for a particle in a fixed location. It takes work to move a particle from a position of lower potential energy to a position of higher potential energy. This increase in potential energy at rest may be converted to (or “realized a ...
Heat Engines, Entropy, and the Second Law of Thermodynamics P
... statement of the second law specifies that the energy removed from the gas to return the temperature to its original value cannot be completely converted to mechanical energy in the form of the work done by the engine in compressing the gas. Thus, we must conclude that the process is irreversible. W ...
... statement of the second law specifies that the energy removed from the gas to return the temperature to its original value cannot be completely converted to mechanical energy in the form of the work done by the engine in compressing the gas. Thus, we must conclude that the process is irreversible. W ...
File
... • The amount of work done, force and distance are related by the equation: work done = force applied × distance moved in direction of force • Work done against frictional forces is mainly transformed into heat. • Elastic potential is the energy stored in an object when work is done on the object to ...
... • The amount of work done, force and distance are related by the equation: work done = force applied × distance moved in direction of force • Work done against frictional forces is mainly transformed into heat. • Elastic potential is the energy stored in an object when work is done on the object to ...
SCIENCE 8
... CW: Chemistry at Work, Journal of Chemical Education PT: Periodic Table, Journal of Chemical Education JCE3: Software Special Issue 3, Journal of Chemical Education ...
... CW: Chemistry at Work, Journal of Chemical Education PT: Periodic Table, Journal of Chemical Education JCE3: Software Special Issue 3, Journal of Chemical Education ...
Chapter 19 Chemical Thermodynamics
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
Chapter 19 Chemical Thermodynamics
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...