MAE 320 – Thermodynamics
... the surrounding is 500 kJ, and the heat transfer from the burner to water is 1500 kJ. Please Note: Qout = 500 kJ and Win = 200 kJ in this question. If student used Qout = 515 kJ and/or Win = 200 kJ , they may have copied the solution from past years. (A) (4p) List the forms of energy transfer involv ...
... the surrounding is 500 kJ, and the heat transfer from the burner to water is 1500 kJ. Please Note: Qout = 500 kJ and Win = 200 kJ in this question. If student used Qout = 515 kJ and/or Win = 200 kJ , they may have copied the solution from past years. (A) (4p) List the forms of energy transfer involv ...
Forms of Energy - Madison County Schools
... • When an object does work on another object, energy is transferred to that object • Power is the rate at which the work is done. ...
... • When an object does work on another object, energy is transferred to that object • Power is the rate at which the work is done. ...
Work Energy Power
... Gravitational potential energy is energy an object possesses because of its position in a gravitational field. The most common use of gravitational potential energy is for an object near the surface of the Earth where the gravitational acceleration can be assumed to be constant at about 9.8 m/s2. Si ...
... Gravitational potential energy is energy an object possesses because of its position in a gravitational field. The most common use of gravitational potential energy is for an object near the surface of the Earth where the gravitational acceleration can be assumed to be constant at about 9.8 m/s2. Si ...
biomolecules and bioenergetics
... • The value of ΔG° alone is, therefore, not sufficient to determine the direction in which a reaction will proceed in a living cell • Even if a reaction is endergonic by virtue of its positive ΔG° , it could be made exergonic by decreasing the mass action ratio, making RT ln [B]/[A] more negative • ...
... • The value of ΔG° alone is, therefore, not sufficient to determine the direction in which a reaction will proceed in a living cell • Even if a reaction is endergonic by virtue of its positive ΔG° , it could be made exergonic by decreasing the mass action ratio, making RT ln [B]/[A] more negative • ...
Chapter 8 Thermochemistry: Chemical Energy
... Standard Heats of Formation Standard Heat of Formation (DHof ): The enthalpy change for the formation of 1 mol of a substance in its standard state from its constituent elements in their ...
... Standard Heats of Formation Standard Heat of Formation (DHof ): The enthalpy change for the formation of 1 mol of a substance in its standard state from its constituent elements in their ...
kinetic energy - Lakeland Regional High School
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
Work and Energy
... In both cases the amount of work done on the system was equal to the change in energy of the system. As it turns out both theorems are a part of a single all encompassing theorem called the work /energy theorem. Where the work done on a system is equal the the change in the total mechanical energy o ...
... In both cases the amount of work done on the system was equal to the change in energy of the system. As it turns out both theorems are a part of a single all encompassing theorem called the work /energy theorem. Where the work done on a system is equal the the change in the total mechanical energy o ...
Slide 1
... Gravitational PE is mgh, where height h is measured relative to some arbitrary reference level where PE = 0. For example, a book on a table has positive PE if the zero reference level is chosen to be the floor. However, if the ceiling is the zero level, then the book has negative PE on the table. It ...
... Gravitational PE is mgh, where height h is measured relative to some arbitrary reference level where PE = 0. For example, a book on a table has positive PE if the zero reference level is chosen to be the floor. However, if the ceiling is the zero level, then the book has negative PE on the table. It ...
Document
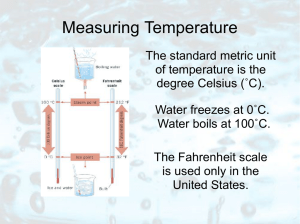
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
Work and Energy In Class Review
... B. An object that moves forever without added energy. C. A change in kinetic energy comes from work. D. Energy can be transformed, but not created nor destroyed. E. Stored work; ability to create forces or cause motion. ...
... B. An object that moves forever without added energy. C. A change in kinetic energy comes from work. D. Energy can be transformed, but not created nor destroyed. E. Stored work; ability to create forces or cause motion. ...
kinetic energy - Batesville Community School
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
kinetic energy - MrcsphysicsWiki
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
... or work is stored when a force does work “against” a force such as the gravitational force or a Hooke’s Law (spring) force. Forces that store or hide energy are ...
Chapter 5 Thermochemistry
... First Law of Thermodynamics • Energy is neither created nor destroyed, but it can undergo a transformation from one type to another. (Law of Conservation of Energy) • The total energy of the universe is a constant. • The energy lost by a system must equal the energy gained by its surroundings, and ...
... First Law of Thermodynamics • Energy is neither created nor destroyed, but it can undergo a transformation from one type to another. (Law of Conservation of Energy) • The total energy of the universe is a constant. • The energy lost by a system must equal the energy gained by its surroundings, and ...
Chapter 3 - Bakersfield College
... B. The amount of work an object performs as it moves toward the earth under the influence of gravity is its gravitational potential energy: PE = mgh where m = mass, g = acceleration of gravity (9.8 m/s2), and h = height. C. The gravitational PE of an object is a relative quantity because it depends ...
... B. The amount of work an object performs as it moves toward the earth under the influence of gravity is its gravitational potential energy: PE = mgh where m = mass, g = acceleration of gravity (9.8 m/s2), and h = height. C. The gravitational PE of an object is a relative quantity because it depends ...
Energy - Effingham County Schools
... Heat – the flow of energy from an object at a higher temperature to an object at a lower temperature. ...
... Heat – the flow of energy from an object at a higher temperature to an object at a lower temperature. ...