File - Physical Science
... Is it possible for one type of energy to change to another type of energy? Consider the picture to the left: At point A, what type of energy does the ball have? Potential Only WHY? At point B, what type of energy does the ball have? Both PE and KE WHY?? At point C, what type of energy does the ball ...
... Is it possible for one type of energy to change to another type of energy? Consider the picture to the left: At point A, what type of energy does the ball have? Potential Only WHY? At point B, what type of energy does the ball have? Both PE and KE WHY?? At point C, what type of energy does the ball ...
Photosynthesis
... process by which light energy is converted to chemical energy in form of glucose. ...
... process by which light energy is converted to chemical energy in form of glucose. ...
Energy types NOTES
... Energy cannot be created or destroyed! Energy can be transferred from one object or another ...
... Energy cannot be created or destroyed! Energy can be transferred from one object or another ...
Physics for semifinal exam 2006-07
... Energy is a conserved quantity, meaning that it cannot be created or destroyed but only converted from one into another. Energy is a scalar quantity because it has no direction in space. The SI unit of energy is the joule(J), equals 1N applied through 1m, for example. The two major categories of ene ...
... Energy is a conserved quantity, meaning that it cannot be created or destroyed but only converted from one into another. Energy is a scalar quantity because it has no direction in space. The SI unit of energy is the joule(J), equals 1N applied through 1m, for example. The two major categories of ene ...
Introduction - WordPress.com
... • The Zeroth Law of Thermodynamics: If two systems are in thermal equilibrium with a third, then they are in thermal equilibrium with each other. This law is the basis of temperature measurement. • First Law of Thermodynamics: The change in internal energy of a closed system is equals to the heat ad ...
... • The Zeroth Law of Thermodynamics: If two systems are in thermal equilibrium with a third, then they are in thermal equilibrium with each other. This law is the basis of temperature measurement. • First Law of Thermodynamics: The change in internal energy of a closed system is equals to the heat ad ...
Electric Potential and Energy
... Any formulae with k in it deals with point charges (individual charges of small radii) so these can NOT be used for problems with parallel plates as there are millions of charges on the plates. ...
... Any formulae with k in it deals with point charges (individual charges of small radii) so these can NOT be used for problems with parallel plates as there are millions of charges on the plates. ...
Section 3. Matter Course Notes
... (f) recall and use the first law of thermodynamics expressed in terms of the increase in internal energy, the heating of the system and the work done on the system. THERMODYNAMIC SYSTEM: For the study of ideal gases, the gas being considered is the system. THE SURROUNDINGS: Everything other than the ...
... (f) recall and use the first law of thermodynamics expressed in terms of the increase in internal energy, the heating of the system and the work done on the system. THERMODYNAMIC SYSTEM: For the study of ideal gases, the gas being considered is the system. THE SURROUNDINGS: Everything other than the ...
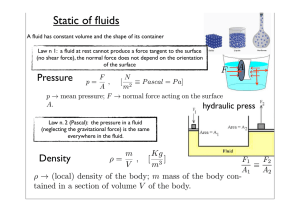
Static of fluids
... is a function of state which can be defined for reversible processes by mens of the heat ∆Qrev exchanged by the system and the corresponding instantaneous temperature T: ∆Qrev ∆S = , [J/K] T ...
... is a function of state which can be defined for reversible processes by mens of the heat ∆Qrev exchanged by the system and the corresponding instantaneous temperature T: ∆Qrev ∆S = , [J/K] T ...
Forms of Energy
... - is the ability to do work or cause change - can be changed from one form to another - cannot be created or destroyed ...
... - is the ability to do work or cause change - can be changed from one form to another - cannot be created or destroyed ...