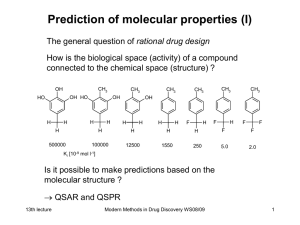
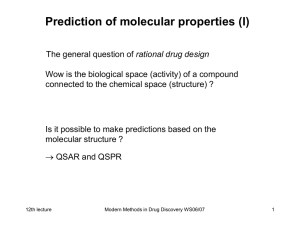
An enquiry into theoretical bioinorganic chemistry: How heuristic is
... judged on the basis of a statistical analysis of results obtained for a test set of molecules. Very many such studies exist and can thus hardly be reviewed here. The problem is that such stochastic statements on the accuracy of density functionals depend on the choice of test molecules and quantitie ...
... judged on the basis of a statistical analysis of results obtained for a test set of molecules. Very many such studies exist and can thus hardly be reviewed here. The problem is that such stochastic statements on the accuracy of density functionals depend on the choice of test molecules and quantitie ...
Density Matrix Calculation of Surface Enhanced
... a valuable vibration-specific spectroscopic tool in spite of lingering questions about the SERS mechanism. Enhancement factors of up to ~106 for molecules on noble metal surfaces were obtained decades ago.1,2 With the recent push toward single-molecule SERS, enhancements of 1012-1014 have been estim ...
... a valuable vibration-specific spectroscopic tool in spite of lingering questions about the SERS mechanism. Enhancement factors of up to ~106 for molecules on noble metal surfaces were obtained decades ago.1,2 With the recent push toward single-molecule SERS, enhancements of 1012-1014 have been estim ...
Low-dimensional weakly interacting Bose gases
... equation of state of a two-dimensional (2D) Bose gas in the < 10−3 been correctly derived [21–23] low density regime na 2 ∼ and checked numerically [24]. In these works the interaction potential is described only by the s-wave scattering length a. So far, no analytical expression for the potential-d ...
... equation of state of a two-dimensional (2D) Bose gas in the < 10−3 been correctly derived [21–23] low density regime na 2 ∼ and checked numerically [24]. In these works the interaction potential is described only by the s-wave scattering length a. So far, no analytical expression for the potential-d ...
chapter 7 - atomic structure
... (1 u = 1.6605 x 10-24 g = 1.6605 x 10-27 kg) Atom contains protons and neutrons that form the nucleus, and electrons occupying the space outside the nucleus. The number of protons (referred to as the atomic number) determines the identity of the atom; neutrons provide nuclear stability and together ...
... (1 u = 1.6605 x 10-24 g = 1.6605 x 10-27 kg) Atom contains protons and neutrons that form the nucleus, and electrons occupying the space outside the nucleus. The number of protons (referred to as the atomic number) determines the identity of the atom; neutrons provide nuclear stability and together ...
1 Path Integrals and Their Application to Dissipative Quantum Systems
... oscillator in Sect. 1.4. Starting from the partition function we will examine several aspects of this dissipative quantum system. Readers interested in a more in-depth treatment of the subject of quantum dissipation are referred to existing textbooks. In particular, we recommend the book by U. Weiss ...
... oscillator in Sect. 1.4. Starting from the partition function we will examine several aspects of this dissipative quantum system. Readers interested in a more in-depth treatment of the subject of quantum dissipation are referred to existing textbooks. In particular, we recommend the book by U. Weiss ...
Electrons in Atoms
... • Chemists found Rutherford’s nuclear model lacking because it did not begin to account for the differences in chemical behavior among the various elements. • In the early 1900s, scientists began to unravel the puzzle of chemical behavior. • They had observed that certain elements emitted visible li ...
... • Chemists found Rutherford’s nuclear model lacking because it did not begin to account for the differences in chemical behavior among the various elements. • In the early 1900s, scientists began to unravel the puzzle of chemical behavior. • They had observed that certain elements emitted visible li ...
09 Electrons in Atoms
... • De Broglie knew that if an electron has wavelike motion and is restricted to circular orbits of fixed radius, the electron is allowed only certain possible wavelengths, frequencies, and energies. • Developing his idea, de Broglie derived an equation for the wavelength (λ) of a particle of mass (m) ...
... • De Broglie knew that if an electron has wavelike motion and is restricted to circular orbits of fixed radius, the electron is allowed only certain possible wavelengths, frequencies, and energies. • Developing his idea, de Broglie derived an equation for the wavelength (λ) of a particle of mass (m) ...
CHAPTER 2 Electron transfer in water and other polar environ
... envisaged in Marcus’ theory. There are two localized electronic states pictured. These are the two redox states. In one, a transferable electron is located at site A; in the other, the transferable charge is located on site B. If the state with the electron at site A is the initial state, species A− ...
... envisaged in Marcus’ theory. There are two localized electronic states pictured. These are the two redox states. In one, a transferable electron is located at site A; in the other, the transferable charge is located on site B. If the state with the electron at site A is the initial state, species A− ...
(4)
... evolution operator is expressed in terms of a quantumclassical bracket have also been studied20–26 and their solutions have been formulated in terms of surface-hopping trajectories.26 –28 In this paper we develop the statistical mechanics of mixed quantum-classical systems. We take as a starting poi ...
... evolution operator is expressed in terms of a quantumclassical bracket have also been studied20–26 and their solutions have been formulated in terms of surface-hopping trajectories.26 –28 In this paper we develop the statistical mechanics of mixed quantum-classical systems. We take as a starting poi ...