... Finally, the nuclear mass, M, has to be mentioned. It satisfies zM < M < 3zM where Mp = 1837m is the mass of a proton. Since the nuclear mass is huge compared to the electron mass, m, it can be considered to be infinite for most purposes, i.e., the nuclei can be regarded as fixed points in R 3 , alt ...
Solved Problems on Quantum Mechanics in One
... Consider a square well having an infinite wall at x = 0 and a wall of height U at x = L. •For the case E < U , obtain solutions to the Schrödinger equation Eq. (20) inside the well (0 ≤ x ≤ L) and in the region beyond (x > L) that satisfy the appropriate boundary conditons at x = 0 and at x = ∞. •E ...
... Consider a square well having an infinite wall at x = 0 and a wall of height U at x = L. •For the case E < U , obtain solutions to the Schrödinger equation Eq. (20) inside the well (0 ≤ x ≤ L) and in the region beyond (x > L) that satisfy the appropriate boundary conditons at x = 0 and at x = ∞. •E ...
Review for Exam 1
... Know the definitions of normalization, orthogonality and orthonormal as they pertains to wavefunctions and eigenfunctions. Be able to perform a normalization and to determine orthogonality as it pertains to wavefunctions. Also you should know how to find the expectation or average value of some quan ...
... Know the definitions of normalization, orthogonality and orthonormal as they pertains to wavefunctions and eigenfunctions. Be able to perform a normalization and to determine orthogonality as it pertains to wavefunctions. Also you should know how to find the expectation or average value of some quan ...
PowerPoint 演示文稿 - Shandong University
... The polar coordinate (r,,) shown in the following is more convenient than the Cartesian coordinate (x,y,z). ...
... The polar coordinate (r,,) shown in the following is more convenient than the Cartesian coordinate (x,y,z). ...
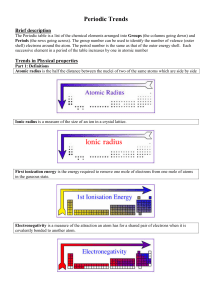
Atomic Structure
... • An atomic orbital is a region of space in which there is a high probability of finding an electron. • Energy levels of electrons are labeld by principal quantum numbers (n). • Each energy sublevel corresponds to an orbital of a different shape, which describes where the electron is likely to be fo ...
... • An atomic orbital is a region of space in which there is a high probability of finding an electron. • Energy levels of electrons are labeld by principal quantum numbers (n). • Each energy sublevel corresponds to an orbital of a different shape, which describes where the electron is likely to be fo ...
Bohr`s model of atom- postulates The electron in an atom moves
... 3.Which of the following orbital has highest energy. (a).n=3 , l=2 ml=+1 (b).n=4 l=0 , ml=0 4.How many unpaired electrons are there in Ni+2? 5.How many electrons in an atom may have the following quantum numbers. (a). n=4 ms=-1/2 (b)n=3 , ml=0 6.An electron is one of the 3d orbital ,Give possible va ...
... 3.Which of the following orbital has highest energy. (a).n=3 , l=2 ml=+1 (b).n=4 l=0 , ml=0 4.How many unpaired electrons are there in Ni+2? 5.How many electrons in an atom may have the following quantum numbers. (a). n=4 ms=-1/2 (b)n=3 , ml=0 6.An electron is one of the 3d orbital ,Give possible va ...
Two-particle systems
... Note on spin: total wave function has to be symmetric or antisymmetric, we have to put together complete two-electron state: ...
... Note on spin: total wave function has to be symmetric or antisymmetric, we have to put together complete two-electron state: ...
Quantum approach - File 2 - College of Science | Oregon State
... Mathematical tools of crucial importance in quantum approach to thermal physics are the density operator op and the mixed state operator M. They are similar, but not identical. Dr. Wasserman in his text, when introducing quantum thermal physics, often “switches” from op to M or vice versa, and ...
... Mathematical tools of crucial importance in quantum approach to thermal physics are the density operator op and the mixed state operator M. They are similar, but not identical. Dr. Wasserman in his text, when introducing quantum thermal physics, often “switches” from op to M or vice versa, and ...
Multi-electron atoms
... Answer is d! Calcium: Fills lowest energy levels first Which orbitals are occupied effects: chemical behavior (bonding, reactivity, etc.) ...
... Answer is d! Calcium: Fills lowest energy levels first Which orbitals are occupied effects: chemical behavior (bonding, reactivity, etc.) ...