teachers.sd43.bc.ca
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
Particle Physics - UW High Energy Physics
... – Interactions of W and Z with higgs field give them mass • Secondary benefit: Yukawa like couplings of matter particles to higgs field give them mass • But, Higgs boson is yet to be found (LHC) May 24, 2017 ...
... – Interactions of W and Z with higgs field give them mass • Secondary benefit: Yukawa like couplings of matter particles to higgs field give them mass • But, Higgs boson is yet to be found (LHC) May 24, 2017 ...
Midterm Review Sheet
... the same proportions by mass regardless of the size of the sample or source of the compound. 3. Law of Multiple Proportions (Dalton) - if two elements can combine to form more than one compound, the masses of the second element that combine with a fixed mass of the first element are in ratios of sma ...
... the same proportions by mass regardless of the size of the sample or source of the compound. 3. Law of Multiple Proportions (Dalton) - if two elements can combine to form more than one compound, the masses of the second element that combine with a fixed mass of the first element are in ratios of sma ...
particle physics
... (1) F(2) = - F(1) (2) Fermi-Dirac statistics (fermions) Note that two Fermions can't be in the same state ...
... (1) F(2) = - F(1) (2) Fermi-Dirac statistics (fermions) Note that two Fermions can't be in the same state ...
Brief introduction to quantum mechanics
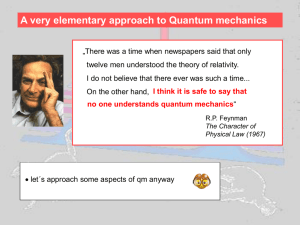
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
Solutions from Yosumism website Problem 41:
... due to user danty.) Moreover, a pi-meson is a hadron. Hadrons interact with the strong-force, and all of them are composed of combinations of quarks. (The fundamental particles are classified as quarks and leptons.) (C) A neutron is made up of 3 quarks. (D) A deuteron consists of a proton and a neut ...
... due to user danty.) Moreover, a pi-meson is a hadron. Hadrons interact with the strong-force, and all of them are composed of combinations of quarks. (The fundamental particles are classified as quarks and leptons.) (C) A neutron is made up of 3 quarks. (D) A deuteron consists of a proton and a neut ...
Lecture 4: Charged Particle Motion
... so when do we need to use the relativistic equations for motion and energy? Well look at the total particle energy (T+m_o c^2), and expand for v/c << 1 ...
... so when do we need to use the relativistic equations for motion and energy? Well look at the total particle energy (T+m_o c^2), and expand for v/c << 1 ...
Chapter 14
... When a neutron is converted into a proton, this is an example of _________. This is the process by which one element changes into another element. Atoms of the same element with a different number of neutrons are called _. The atomic number of an element is equal to _____________. This is the smalle ...
... When a neutron is converted into a proton, this is an example of _________. This is the process by which one element changes into another element. Atoms of the same element with a different number of neutrons are called _. The atomic number of an element is equal to _____________. This is the smalle ...
Goal 4.01
... Rutherford concluded that the space was small due to how few alpha particles were deflected. Rutherford named the small, positively charged space a nucleus and the space surrounding it an electron cloud. He named the particles inside the nucleus that gave it it’s positive charge protons. This positi ...
... Rutherford concluded that the space was small due to how few alpha particles were deflected. Rutherford named the small, positively charged space a nucleus and the space surrounding it an electron cloud. He named the particles inside the nucleus that gave it it’s positive charge protons. This positi ...
s2o1d
... C. been found in underwater canyons D. been sorted by size and density 2. A streambed contains round rocks, all about the same size. Why are there no smaller particles of sand and clay? Sand and clay A. are too small to see B. are denser C. have washed away D. were never there 3. A lake study reveal ...
... C. been found in underwater canyons D. been sorted by size and density 2. A streambed contains round rocks, all about the same size. Why are there no smaller particles of sand and clay? Sand and clay A. are too small to see B. are denser C. have washed away D. were never there 3. A lake study reveal ...
Section 1 Review
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
Particle Sizing
... DLS: dynamic light scattering QELS: quasi-elastic light scattering PCS: photon correlation spectroscopy HDC: hydrodynamic chromatography ...
... DLS: dynamic light scattering QELS: quasi-elastic light scattering PCS: photon correlation spectroscopy HDC: hydrodynamic chromatography ...
The Nobel Prize in Physics 2004
... in spite of the very large difference in strength between the two interactions there are several similarities. The interaction strength decreases with the square of the distance and has a long range. Both the electromagnetic interaction and the gravitational interaction are mediated by force carrie ...
... in spite of the very large difference in strength between the two interactions there are several similarities. The interaction strength decreases with the square of the distance and has a long range. Both the electromagnetic interaction and the gravitational interaction are mediated by force carrie ...
Particle Physics
... • Protons/nuclei can look point-like under many experimental conditions • Atoms/molecules can look point-like to a typical human QFT can be used to describe any such system… … it has nothing to do with the system being “fundamental” But QFT becomes essential when the energies are such that particles ...
... • Protons/nuclei can look point-like under many experimental conditions • Atoms/molecules can look point-like to a typical human QFT can be used to describe any such system… … it has nothing to do with the system being “fundamental” But QFT becomes essential when the energies are such that particles ...
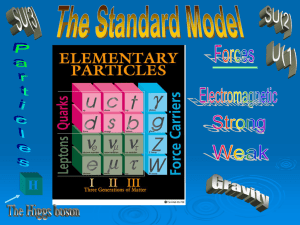
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.