Chapter 30 - Planet Holloway
... Is responsible for the binding of atoms and molecules About 10-2 times the strength of the strong force A long-range force that decreases in strength as the inverse square of the separation between interacting particles ...
... Is responsible for the binding of atoms and molecules About 10-2 times the strength of the strong force A long-range force that decreases in strength as the inverse square of the separation between interacting particles ...
quantum1
... Given the Uncertainty Principle, how do you write an equation of motion for a particle? •First, remember that a particle is only a particle sort of, and a wave sort of, and it’s not quite like anything you’ve encountered in classical physics. We need to use Fourier’s Theorem to represent the parti ...
... Given the Uncertainty Principle, how do you write an equation of motion for a particle? •First, remember that a particle is only a particle sort of, and a wave sort of, and it’s not quite like anything you’ve encountered in classical physics. We need to use Fourier’s Theorem to represent the parti ...
Particle Identification in High Energy Physics
... How Much Energy? Higgs? Supersymmetry? top quark W§/Z0 bosons charm and bottom quarks anti-proton production threshold kaon production threshold pion production threshold positron production threshold x-rays: Roentgen, 1895 ...
... How Much Energy? Higgs? Supersymmetry? top quark W§/Z0 bosons charm and bottom quarks anti-proton production threshold kaon production threshold pion production threshold positron production threshold x-rays: Roentgen, 1895 ...
Exam on Matter through Bonding
... 11. Which of these types of nuclear radiation has the greatest penetrating power? (1) alpha (3) neutron (2) beta (4) gamma 12. Alpha particles and beta particles differ in (1) mass, only (2) charge, only (3) both mass and charge (4) neither mass nor charge 13. Which equation represents a fusion reac ...
... 11. Which of these types of nuclear radiation has the greatest penetrating power? (1) alpha (3) neutron (2) beta (4) gamma 12. Alpha particles and beta particles differ in (1) mass, only (2) charge, only (3) both mass and charge (4) neither mass nor charge 13. Which equation represents a fusion reac ...
Quantum Mechanics: Introduction
... 3. Position and momentum of a particle cannot be measured accurately simultaneously. (Heisenberg uncertainty principle) 4. Energy of wave is related with frequency and quantised. ...
... 3. Position and momentum of a particle cannot be measured accurately simultaneously. (Heisenberg uncertainty principle) 4. Energy of wave is related with frequency and quantised. ...
Alessandro Bettini Introduction to Elementary Particle Physics
... is the d band ? Why is it so far to the right ? ...
... is the d band ? Why is it so far to the right ? ...
DirectProducts
... 1999 Gerardus ‘t Hooft and Martinus J. G. Veltman Renormalization theories of electroweak interactions ...
... 1999 Gerardus ‘t Hooft and Martinus J. G. Veltman Renormalization theories of electroweak interactions ...
FINAL REVIEW 1st SEMESTER 2014-2015
... Balance the following equation. aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
... Balance the following equation. aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
Charges in a Magnetic Field
... • Between 1909-1913, Robert Millikin found the charge for an electron using his oil drop ...
... • Between 1909-1913, Robert Millikin found the charge for an electron using his oil drop ...
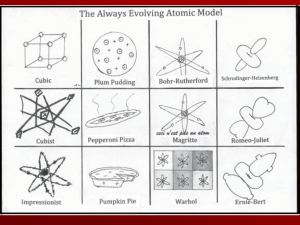
The Structure of an Atom
... The number of protons in an atom is balanced by the number of electrons in the atom so that the atom does not carry a net charge So, the atomic number also tells us the # of electrons ...
... The number of protons in an atom is balanced by the number of electrons in the atom so that the atom does not carry a net charge So, the atomic number also tells us the # of electrons ...
Formation of planetesimals in collapsing particle clouds
... In the cloud, particles would move around and collide with each other. Collisions dissipate away energy from the cloud (unless they are completely elastic) and increase the binding energy. Due to the negative heat capacity nature of a self-gravitating system the cloud ‘heats’ up when it loses energy ...
... In the cloud, particles would move around and collide with each other. Collisions dissipate away energy from the cloud (unless they are completely elastic) and increase the binding energy. Due to the negative heat capacity nature of a self-gravitating system the cloud ‘heats’ up when it loses energy ...
Chapter 30: Nuclear Energy and Elementary Particles
... Electromagnetic force Weak force Gravitational force ...
... Electromagnetic force Weak force Gravitational force ...
∑ ∑
... all particles move with the same speed. Many applications, however, require a beam in which all the particle speeds are the same. Using crossed fields particles of a specific speed can be selected as follows: We can use the magnetic force in conjunction with the electric force to filter out particle ...
... all particles move with the same speed. Many applications, however, require a beam in which all the particle speeds are the same. Using crossed fields particles of a specific speed can be selected as follows: We can use the magnetic force in conjunction with the electric force to filter out particle ...
AtomicStructure
... Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. ...
... Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. ...
Conservation of Momentum Exercise
... A particle of charge q travelling at right‐angles to a magnetic field B with a speed v experiences a force Bqv at right angles to its motion. This makes the particle follow a circular path of radius r and the motion is described by Bqv = mv2/r → p = (Bq) r This tells us that for a fixed field B, and ...
... A particle of charge q travelling at right‐angles to a magnetic field B with a speed v experiences a force Bqv at right angles to its motion. This makes the particle follow a circular path of radius r and the motion is described by Bqv = mv2/r → p = (Bq) r This tells us that for a fixed field B, and ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.