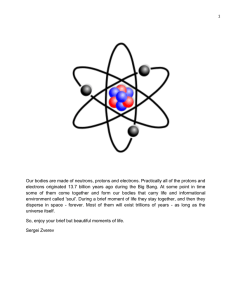
Quantum Field Theory - Why and When?
... space of the Hilbert spaces of all the constituents. A problem occurs when we attempt to describe processes where the number of particles changes as a result of the interactions. The example of atom formation from nuclei and electrons, alluded to above, is a good illustration. When an electron is ca ...
... space of the Hilbert spaces of all the constituents. A problem occurs when we attempt to describe processes where the number of particles changes as a result of the interactions. The example of atom formation from nuclei and electrons, alluded to above, is a good illustration. When an electron is ca ...
What`s Inside the Nucleus?
... • The Standard Model of nuclear and particle physics has been superbly successful, but is now looking a bit frayed around the edges. And it has never really worked in the world we live in with protons and neutrons and atomic nuclei. ...
... • The Standard Model of nuclear and particle physics has been superbly successful, but is now looking a bit frayed around the edges. And it has never really worked in the world we live in with protons and neutrons and atomic nuclei. ...
Gravitational potential energy for a particle near the surface of the
... however, it has the same features as we expect for gravitational potential energy–it is large for large separation and it is small for small separation. Thus, it is directly proportional to the separation of the particles (particle and Earth). ...
... however, it has the same features as we expect for gravitational potential energy–it is large for large separation and it is small for small separation. Thus, it is directly proportional to the separation of the particles (particle and Earth). ...
Element: pure substances that are made up of one kind of atom
... Element: pure substances that are made up of one kind of atom. Elements are abbreviated in scientific shorthand. They are written with either one capital letter, or a capital letter and a lowercase letter. These abbreviations are called a ...
... Element: pure substances that are made up of one kind of atom. Elements are abbreviated in scientific shorthand. They are written with either one capital letter, or a capital letter and a lowercase letter. These abbreviations are called a ...
Electrons exhibit both wave
... his model of the atom as a central massive positive nucleus, with large amounts of empty space in which the small negative electrons can be found. He proposed that the electrons are held in place by the electrostatic attraction between the electron and nucleus providing the central force. However, c ...
... his model of the atom as a central massive positive nucleus, with large amounts of empty space in which the small negative electrons can be found. He proposed that the electrons are held in place by the electrostatic attraction between the electron and nucleus providing the central force. However, c ...
MatterPP4
... energy level determines the chemical behavior of the different elements. Valence electrons are the outermost electrons in an atom. Elements with the same number of valence electrons have similar chemical properties. Group 1 has 1 valence electron, group 2 has 2, 13 has 3, 14 / 4, 15 / 5, 16 / 6, 17 ...
... energy level determines the chemical behavior of the different elements. Valence electrons are the outermost electrons in an atom. Elements with the same number of valence electrons have similar chemical properties. Group 1 has 1 valence electron, group 2 has 2, 13 has 3, 14 / 4, 15 / 5, 16 / 6, 17 ...
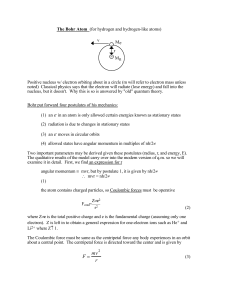
F = mv r
... Positive nucleus w/ electron orbiting about in a circle (m will refer to electron mass unless noted) Classical physics says that the electron will radiate (lose energy) and fall into the nucleus, but it doesn't. Why this is so is answered by "old" quantum theory. Bohr put forward four postulates of ...
... Positive nucleus w/ electron orbiting about in a circle (m will refer to electron mass unless noted) Classical physics says that the electron will radiate (lose energy) and fall into the nucleus, but it doesn't. Why this is so is answered by "old" quantum theory. Bohr put forward four postulates of ...
Quanta to Quarks part 2 - Connecting-Sharing-and
... been slowed down to thermal velocities by moderator material, and undergoes fission, which releases heat energy. Control rods containing neutron-absorbing material are used to control the rate of reaction. The heat produced is absorbed by a coolant material and can be transferred via a series of hea ...
... been slowed down to thermal velocities by moderator material, and undergoes fission, which releases heat energy. Control rods containing neutron-absorbing material are used to control the rate of reaction. The heat produced is absorbed by a coolant material and can be transferred via a series of hea ...
radioactivity PowerPoint Presentation
... • Nucleus changes mass by four units and charge by two units – Alpha particle easily stopped – 4 x nucleon mass ...
... • Nucleus changes mass by four units and charge by two units – Alpha particle easily stopped – 4 x nucleon mass ...
Homework Book
... A nucleus of large mass number splits into two nuclei, releasing several electrons. ...
... A nucleus of large mass number splits into two nuclei, releasing several electrons. ...
Honors
... 2. Choose a synthetic element and write a biography of the element. Include information about the element’s name, location of the synthesis research, and the people responsible. 3. Research and report on current theorized subatomic particle, such as quarks, leptons, and gluons. (You may make a poste ...
... 2. Choose a synthetic element and write a biography of the element. Include information about the element’s name, location of the synthesis research, and the people responsible. 3. Research and report on current theorized subatomic particle, such as quarks, leptons, and gluons. (You may make a poste ...
Review PH301 -- duality, wavefunction, probability
... OR you can think of wavefucntion as having two components like light has E-field and B-field each component will be real but you will have two components to calculate with two coupled differential eqns complex functions make the math easier! ...
... OR you can think of wavefucntion as having two components like light has E-field and B-field each component will be real but you will have two components to calculate with two coupled differential eqns complex functions make the math easier! ...
Asymptotic Freedom: From Paradox to Paradigm 1 A Pair of Paradoxes ∗
... two great theories of twentieth-century physics. Both are very successful. But these two theories are based on entirely different ideas, which are not easy to reconcile. In particular, special relativity puts space and time on the same footing, but quantum mechanics treats them very differently. Thi ...
... two great theories of twentieth-century physics. Both are very successful. But these two theories are based on entirely different ideas, which are not easy to reconcile. In particular, special relativity puts space and time on the same footing, but quantum mechanics treats them very differently. Thi ...
Asymptotic Freedom: From Paradox to Paradigm
... two great theories of twentieth-century physics. Both are very successful. But these two theories are based on entirely different ideas, which are not easy to reconcile. In particular, special relativity puts space and time on the same footing, but quantum mechanics treats them very differently. Thi ...
... two great theories of twentieth-century physics. Both are very successful. But these two theories are based on entirely different ideas, which are not easy to reconcile. In particular, special relativity puts space and time on the same footing, but quantum mechanics treats them very differently. Thi ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.