Electrons - SwissEduc
... have been no more surprised if someone had fired a 15-inch artillery shell into tissue paper and then found it in flight back toward the cannon. What allowed most of the alpha particles to pass through the gold foil in a rather straight path? According to Rutherford’s interpretation, the atom is mos ...
... have been no more surprised if someone had fired a 15-inch artillery shell into tissue paper and then found it in flight back toward the cannon. What allowed most of the alpha particles to pass through the gold foil in a rather straight path? According to Rutherford’s interpretation, the atom is mos ...
Cathode Rays
... been no more surprised if someone had fired a 15-inch artillery shell into tissue paper and then found it in flight back toward the cannon. What allowed most of the alpha particles to pass through the gold foil in a rather straight path? According to Rutherford’s interpretation, the atom is mostly e ...
... been no more surprised if someone had fired a 15-inch artillery shell into tissue paper and then found it in flight back toward the cannon. What allowed most of the alpha particles to pass through the gold foil in a rather straight path? According to Rutherford’s interpretation, the atom is mostly e ...
CHEM 121
... Similarly, if you have photons with sufficient energy to eject an electron, each photon can only eject one electron, and you need more photons (more intense light) to eject more electrons. The greater the photon energy is beyond the threshold energy, the more kinetic energy an ejected electron can h ...
... Similarly, if you have photons with sufficient energy to eject an electron, each photon can only eject one electron, and you need more photons (more intense light) to eject more electrons. The greater the photon energy is beyond the threshold energy, the more kinetic energy an ejected electron can h ...
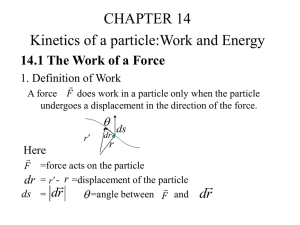
here
... Derivation of ideal gas equations leading to Boyle’s law and Avogadro’s hypothesis. p = 1/3 n vm < c2 >; temperature defined by pVm = RT; p = n vkT. (see 9.3) The Avogadro constant [see 2(b)]. A simplified treatment (e.g. particles in a rectangular box with statistics treated by dividing the molecul ...
... Derivation of ideal gas equations leading to Boyle’s law and Avogadro’s hypothesis. p = 1/3 n vm < c2 >; temperature defined by pVm = RT; p = n vkT. (see 9.3) The Avogadro constant [see 2(b)]. A simplified treatment (e.g. particles in a rectangular box with statistics treated by dividing the molecul ...
Sonication
... disruptions can be used to mix solutions, speed the dissolution of a solid into a liquid (like sugar into water), and remove dissolved gas from liquids. Sound is a wave made up of alternating regions of high and low pressure. 1 Imagine yourself as a particle. As a sound wave passes you, you experien ...
... disruptions can be used to mix solutions, speed the dissolution of a solid into a liquid (like sugar into water), and remove dissolved gas from liquids. Sound is a wave made up of alternating regions of high and low pressure. 1 Imagine yourself as a particle. As a sound wave passes you, you experien ...
Lecture11(CavitiesI) 2015 - Indico
... in resonant cavity and particles enter and leave by holes in end walls. Energy is continuously exchanged between electric and magnetic fields within cavity volume. The time-varying fields ensure finite energy increment at each passage through one or a chain of cavities. There is no build-up of volta ...
... in resonant cavity and particles enter and leave by holes in end walls. Energy is continuously exchanged between electric and magnetic fields within cavity volume. The time-varying fields ensure finite energy increment at each passage through one or a chain of cavities. There is no build-up of volta ...
The exotic world of quantum matter
... Majorana fermions at the core of vortices in He3-B Superfluid He3-B is a topologically non-trivial superfluid, supporting ring vortices of vorticity 1 (winding of the R-matrix) In the vortex core fermionic excitations may exist, which appear in time-reversal invariant pairs (Dirac) If the rings are ...
... Majorana fermions at the core of vortices in He3-B Superfluid He3-B is a topologically non-trivial superfluid, supporting ring vortices of vorticity 1 (winding of the R-matrix) In the vortex core fermionic excitations may exist, which appear in time-reversal invariant pairs (Dirac) If the rings are ...
Static Electricity
... every other electron so they all have the same mass and the same negative charge • The nucleus is composed of positively charged protons and uncharged neutrons • All protons are identical and the charge of the proton is exactly the same size as the charge of the electron, but it is opposite ...
... every other electron so they all have the same mass and the same negative charge • The nucleus is composed of positively charged protons and uncharged neutrons • All protons are identical and the charge of the proton is exactly the same size as the charge of the electron, but it is opposite ...
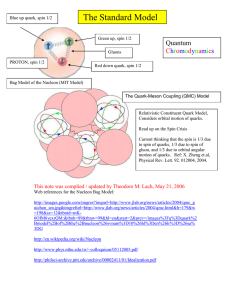
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.