Beam and detectors - A Beamline for Schools
... Signals from some of the detectors are used to build the trigger. The trigger logic identifies interesting interactions (“events”) and instructs the computer to initiate the readout of the data from all the detectors. The trigger is a fundamental and complex component of LHC experiments, where colli ...
... Signals from some of the detectors are used to build the trigger. The trigger logic identifies interesting interactions (“events”) and instructs the computer to initiate the readout of the data from all the detectors. The trigger is a fundamental and complex component of LHC experiments, where colli ...
15-1. principle of linear impulse and momentum
... The forces are very large , act for a very short period of time and produce a significant charge in momentum. Note :when making the distinction between impulsive and nonimpulsive forces it is important to realize that it only applied during a specific time. Conservation of linear moment. The vector ...
... The forces are very large , act for a very short period of time and produce a significant charge in momentum. Note :when making the distinction between impulsive and nonimpulsive forces it is important to realize that it only applied during a specific time. Conservation of linear moment. The vector ...
atomic number - Southwest High School
... study of the atom began with John Dalton in the early 1800’s. ...
... study of the atom began with John Dalton in the early 1800’s. ...
Lecture-2: Atomic Structure
... from a metal surface when light shines on it. The discovery of the photoelectric effect could not be explained by the electromagnetic theory of light. Albert Einstein developed the quantum theory of light in ...
... from a metal surface when light shines on it. The discovery of the photoelectric effect could not be explained by the electromagnetic theory of light. Albert Einstein developed the quantum theory of light in ...
Pressure is explained by kinetic theory as arising from
... where V=L3 is the volume of the box. The fraction n=N/V is the number density of the gas. This is a first non-trivial result of the kinetic theory because it relates pressure (a macroscopic property) to the average (translational) kinetic energy per molecule which is a microscopic ...
... where V=L3 is the volume of the box. The fraction n=N/V is the number density of the gas. This is a first non-trivial result of the kinetic theory because it relates pressure (a macroscopic property) to the average (translational) kinetic energy per molecule which is a microscopic ...
Chapter 3. The Structure of the Atom
... (3.31) yields information on Z 2 the charge of the nucleus, while the experimental determination of the scattering angles confirmed that it is massive and concentrated). Although these results clearly excluded Thomson’s plum-pudding model, it did not completely rule out a classical model. Indeed, Th ...
... (3.31) yields information on Z 2 the charge of the nucleus, while the experimental determination of the scattering angles confirmed that it is massive and concentrated). Although these results clearly excluded Thomson’s plum-pudding model, it did not completely rule out a classical model. Indeed, Th ...
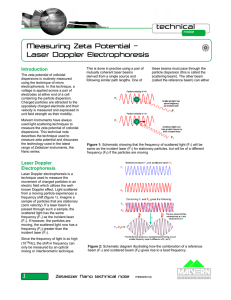
Measuring Zeta Potential – Laser Doppler Electrophoresis
... This is done in practice using a pair of mutually coherent laser beams derived from a single source and following similar path lengths. One of ...
... This is done in practice using a pair of mutually coherent laser beams derived from a single source and following similar path lengths. One of ...
Prior knowledge catch-up student sheet for Chapter 3 Quantitative
... the equation. This is because atoms cannot be created or destroyed. As there are the same atoms present at the start and end of a chemical reaction, mass must be conserved in the reaction. In other words, the total mass of the reactants must equal the total mass of the products. This is known as the ...
... the equation. This is because atoms cannot be created or destroyed. As there are the same atoms present at the start and end of a chemical reaction, mass must be conserved in the reaction. In other words, the total mass of the reactants must equal the total mass of the products. This is known as the ...
Physics 30 - Structured Independent Learning
... The search for anti-matter. At the beginning of time, 13.7 billion years ago, it is believed that an extremely dense ball of energy, small than an atom, exploded and sent high energy cosmic particles flying out in all directions. These particles were the origins of what is now known as the UNIVERSE. ...
... The search for anti-matter. At the beginning of time, 13.7 billion years ago, it is believed that an extremely dense ball of energy, small than an atom, exploded and sent high energy cosmic particles flying out in all directions. These particles were the origins of what is now known as the UNIVERSE. ...
Chapter 2 - Chemistry
... 4.) Law of Multiple Proportions - when two elements form more than one compound, the masses of one element in these compounds for a fixed mass of the other element are in ratios of small whole numbers Karen Hattenhauer (Fall 2007) ...
... 4.) Law of Multiple Proportions - when two elements form more than one compound, the masses of one element in these compounds for a fixed mass of the other element are in ratios of small whole numbers Karen Hattenhauer (Fall 2007) ...
information
... Significance evidence shown in past SPS and offsite experiments but details still need to be confirmed.. ...
... Significance evidence shown in past SPS and offsite experiments but details still need to be confirmed.. ...
Physics 30 - Structured Independent Learning
... The search for anti-matter. At the beginning of time, 13.7 billion years ago, it is believed that an extremely dense ball of energy, small than an atom, exploded and sent high energy cosmic particles flying out in all directions. These particles were the origins of what is now known as the UNIVERSE. ...
... The search for anti-matter. At the beginning of time, 13.7 billion years ago, it is believed that an extremely dense ball of energy, small than an atom, exploded and sent high energy cosmic particles flying out in all directions. These particles were the origins of what is now known as the UNIVERSE. ...
Chapter 4 Assignment Answers
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
Conservation Laws I - Department of Physics, HKU
... While the maths behind Noether’s theorem is difficult the general idea can be understood with respect to some imaginary universes: (a) In this universe laws are not constant in time. As time progresses electric charges increase with time. The spring shown compresses – building potential energy (not ...
... While the maths behind Noether’s theorem is difficult the general idea can be understood with respect to some imaginary universes: (a) In this universe laws are not constant in time. As time progresses electric charges increase with time. The spring shown compresses – building potential energy (not ...
Measuring the Size of Elementary Particle Collisions
... Bosons are integer spin particles. Identical Bosons have a symmetric two particle wave function -any number may occupy a given quantum state... Photons and pions are examples of Bosons Fermions are half-integer spin particles. Identical Fermions have an antisymmetric wave function -only one particle ...
... Bosons are integer spin particles. Identical Bosons have a symmetric two particle wave function -any number may occupy a given quantum state... Photons and pions are examples of Bosons Fermions are half-integer spin particles. Identical Fermions have an antisymmetric wave function -only one particle ...
Presentation
... • The period of development of the old quantum theory (roughly 1900-1923) was a heroic period, characterized by trials and errors. • The discovery of the nucleus and the introduction of quantized orbits - a major milestone in the middle of the period - worthy of being celebrated. • Both Rutherford a ...
... • The period of development of the old quantum theory (roughly 1900-1923) was a heroic period, characterized by trials and errors. • The discovery of the nucleus and the introduction of quantized orbits - a major milestone in the middle of the period - worthy of being celebrated. • Both Rutherford a ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.