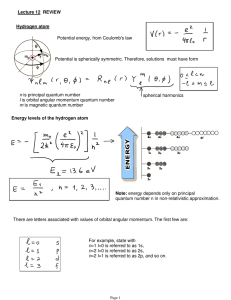
Lecture 12: Review.
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
lecture 10
... Consider a particle of mass m moving along positive x-axis. Particle is said to be free if it is not under the influence of any field or force. Therefore for a free particle potential energy can be considered to be constant or zero. The Schrodinger wave equation for a free particle is given by. ...
... Consider a particle of mass m moving along positive x-axis. Particle is said to be free if it is not under the influence of any field or force. Therefore for a free particle potential energy can be considered to be constant or zero. The Schrodinger wave equation for a free particle is given by. ...
Effect of Generation of Charged Particles Fluxes
... Ions accelerated to the energy above 100 keV can enter nuclear reactions when they collide with nuclei of atoms that compose air. Some reports on observations of generation of fluxes of heavy charged particles during thunderstorms are known. Sources of dense fluxes of electrons similar to the source ...
... Ions accelerated to the energy above 100 keV can enter nuclear reactions when they collide with nuclei of atoms that compose air. Some reports on observations of generation of fluxes of heavy charged particles during thunderstorms are known. Sources of dense fluxes of electrons similar to the source ...
Chemistry I – Semester I Final Review
... Define and apply the Law of Conservation of Mass - define atom, nucleus, electron, neutron, isotope, proton (including relative size and charge of subatomic particles, average atomic mass, cation, anion, atomic number, atomic mass - discuss the evolution of atomic theory to the present electron clou ...
... Define and apply the Law of Conservation of Mass - define atom, nucleus, electron, neutron, isotope, proton (including relative size and charge of subatomic particles, average atomic mass, cation, anion, atomic number, atomic mass - discuss the evolution of atomic theory to the present electron clou ...
Total Notes for chem - Catawba County Schools
... means that they can be split into smaller pieces). There are over two hundred known particles in this category. QUARKS- Relatively large particles which make up protons and electrons. There are six kinds ( or flavors !) of quark. They are TRUTH(top),Beauty(bottom),UP,DOWN,Strange,and Charm.(or CHARM ...
... means that they can be split into smaller pieces). There are over two hundred known particles in this category. QUARKS- Relatively large particles which make up protons and electrons. There are six kinds ( or flavors !) of quark. They are TRUTH(top),Beauty(bottom),UP,DOWN,Strange,and Charm.(or CHARM ...
transparencies - Indico
... among known physical quantities: masses, forces These are theories we can experimentally test ...
... among known physical quantities: masses, forces These are theories we can experimentally test ...
Charging of positively charged dust particle in weak magnetic field*
... Absorption cross-section of a spherical dust particle with positive charge by an electron/ion is studied in weak uniform magnetic field. The absorption cross-section in the absence of magnetic field was expressed by the OML (Orbit Motion Limited) theory [1, 2]. The OML theory, where energy and angul ...
... Absorption cross-section of a spherical dust particle with positive charge by an electron/ion is studied in weak uniform magnetic field. The absorption cross-section in the absence of magnetic field was expressed by the OML (Orbit Motion Limited) theory [1, 2]. The OML theory, where energy and angul ...
Radioactive Decay
... • Radioactivity: Substances spontaneously emit radiation • Radiation: rays and particles emitted by radioactive material • Radioactive atoms go through changes that alter their identity – aka changes from one atom to another • How can this happen? ...
... • Radioactivity: Substances spontaneously emit radiation • Radiation: rays and particles emitted by radioactive material • Radioactive atoms go through changes that alter their identity – aka changes from one atom to another • How can this happen? ...
Sections 3 - Columbia Physics
... Consider a two-level quantum system described by Hamiltonian H, with states |a> and |b> and energies Ea = 0 and Eb = E0. The system is initially in state |a>. Suppose that a constant perturbation H’ is applied from time t = 0 until some arbitrary subsequent time t. Find the probability Pb(t) of bein ...
... Consider a two-level quantum system described by Hamiltonian H, with states |a> and |b> and energies Ea = 0 and Eb = E0. The system is initially in state |a>. Suppose that a constant perturbation H’ is applied from time t = 0 until some arbitrary subsequent time t. Find the probability Pb(t) of bein ...
The Two-Body problem
... i.e no explicit time dependence of L - time translation invariance. This implies that total energy, E= ...
... i.e no explicit time dependence of L - time translation invariance. This implies that total energy, E= ...
1-d examples
... 2) Ground state energy is not zero! (i.e., it is above the bottom of the potential well). This is a general feature of QM. Here, it can be heuristically justified by invoking the uncertainty principle. 3) Note that the ground state eigenfunction has no nodes (disregarding the boundaries); the first ...
... 2) Ground state energy is not zero! (i.e., it is above the bottom of the potential well). This is a general feature of QM. Here, it can be heuristically justified by invoking the uncertainty principle. 3) Note that the ground state eigenfunction has no nodes (disregarding the boundaries); the first ...
Atomic Structure
... The nucleus (the positively-charged center of the atom) is held together by a strong nuclear force, which “glues” protons together. The electrons are attracted to the nucleus by an electromagnetic force. This holds the atom together. Gravity plays a part, too, but is the weakest of the forces in nat ...
... The nucleus (the positively-charged center of the atom) is held together by a strong nuclear force, which “glues” protons together. The electrons are attracted to the nucleus by an electromagnetic force. This holds the atom together. Gravity plays a part, too, but is the weakest of the forces in nat ...
Modern Physics
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
Document
... carrying current i may be taken as B0. If a particle of charge q passes the centre of a semicircular wire, as shown below, along the axis of the wire, the force on it due to the current (with v as the particle speed) is. ...
... carrying current i may be taken as B0. If a particle of charge q passes the centre of a semicircular wire, as shown below, along the axis of the wire, the force on it due to the current (with v as the particle speed) is. ...
Okada
... in supersymmetric model The Standard Model of elementary particle physics The best theory we know so far in describing elementary particle physics @ E=O(100 GeV) ...
... in supersymmetric model The Standard Model of elementary particle physics The best theory we know so far in describing elementary particle physics @ E=O(100 GeV) ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.