Thermodynamics 1. Refer to the following
... The student measures the time it takes for the piece of wax on each block to melt. Which of the following questions is being studied in this experiment? A. What is the melting point of the wax? B. What is the melting point of each metal? C. Which metal radiates heat the best? D. Which metal conducts ...
... The student measures the time it takes for the piece of wax on each block to melt. Which of the following questions is being studied in this experiment? A. What is the melting point of the wax? B. What is the melting point of each metal? C. Which metal radiates heat the best? D. Which metal conducts ...
Thermochem
... Sometimes it is hard to measure the change for a reaction. If this is the case, you can calculate the enthalpy change of the reaction from standard heats of formation. Standard heat of formation (Hof) is the change in enthalpy that accompanies the formation of one mole of the compound from its elem ...
... Sometimes it is hard to measure the change for a reaction. If this is the case, you can calculate the enthalpy change of the reaction from standard heats of formation. Standard heat of formation (Hof) is the change in enthalpy that accompanies the formation of one mole of the compound from its elem ...
Thermodynamic functions - Phase Transformations Group
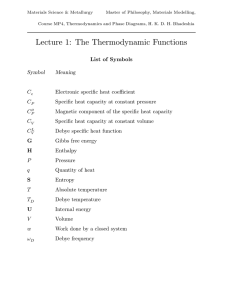
... such as H, G and S are path independent and therefore are called functions of state. More About the Heat Capacity The heat capacity can be determined experimentally using calorimetry. The data can then be related directly to the functions of state H, G and S. The heat capacity varies with temperatur ...
... such as H, G and S are path independent and therefore are called functions of state. More About the Heat Capacity The heat capacity can be determined experimentally using calorimetry. The data can then be related directly to the functions of state H, G and S. The heat capacity varies with temperatur ...
Week 11 - Guelph Physics
... permeable to glycerol and water. A solution of 0.15 molar NaCL is isotonic for this type of cell. Will a solution of 0.30 molar glycerol be isotonic, hypotonic or hypertonic for this kind of cell? ...
... permeable to glycerol and water. A solution of 0.15 molar NaCL is isotonic for this type of cell. Will a solution of 0.30 molar glycerol be isotonic, hypotonic or hypertonic for this kind of cell? ...
First law of thermodynamics
... Second law of thermodynamics Before look at the second law of thermodynamics, let us discuss where first law of thermodynamics fails. According to the first law of thermodynamics, whenever any process occurs, there may be either heat interaction or work interaction. But we cannot exactly tell by fir ...
... Second law of thermodynamics Before look at the second law of thermodynamics, let us discuss where first law of thermodynamics fails. According to the first law of thermodynamics, whenever any process occurs, there may be either heat interaction or work interaction. But we cannot exactly tell by fir ...
First Law of Thermodynamics - Erwin Sitompul
... thermodynamic process, where: Heat Q can be added to the gas (positive heat) or withdrawn from it (negative heat), by regulating the temperature of the adjustable ...
... thermodynamic process, where: Heat Q can be added to the gas (positive heat) or withdrawn from it (negative heat), by regulating the temperature of the adjustable ...
Thermodynamics - SeyedAhmad.com
... the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating ...
... the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating ...
TechTopics No. 74 - Heat generation estimation for type
... will have heat generation only one-quarter of that at full-rated continuous current. Because the effect of the square relationship is very significant, it is overly conservative to estimate heat generation based on the assumption that all sections and all circuit breakers each carry their rated cont ...
... will have heat generation only one-quarter of that at full-rated continuous current. Because the effect of the square relationship is very significant, it is overly conservative to estimate heat generation based on the assumption that all sections and all circuit breakers each carry their rated cont ...
Print - Advances in Physiology Education
... conductor can be used several times over to transfer heat; a combustible rod has to be replaced after every episode of heat transfer. 3) The heat transfer is much faster in conductors. 4) The heat transfer by combustion can be viewed as a high-fidelity transmission (the temperature of the flame rema ...
... conductor can be used several times over to transfer heat; a combustible rod has to be replaced after every episode of heat transfer. 3) The heat transfer is much faster in conductors. 4) The heat transfer by combustion can be viewed as a high-fidelity transmission (the temperature of the flame rema ...
teaching nerve conduction to undergraduates
... conductor can be used several times over to transfer heat; a combustible rod has to be replaced after every episode of heat transfer. 3) The heat transfer is much faster in conductors. 4) The heat transfer by combustion can be viewed as a high-fidelity transmission (the temperature of the flame rema ...
... conductor can be used several times over to transfer heat; a combustible rod has to be replaced after every episode of heat transfer. 3) The heat transfer is much faster in conductors. 4) The heat transfer by combustion can be viewed as a high-fidelity transmission (the temperature of the flame rema ...
Thermodynamics Exam 1 Info/Problems
... 17. temperature, pressure, density, entropy per mass, internal energy per mass. 18. The ball would likely pass through easily. 19. The strip would bend, because the two metals will expand at different rates. 20. The glass is also expanding, which would cause it to look like the liquid is expanding l ...
... 17. temperature, pressure, density, entropy per mass, internal energy per mass. 18. The ball would likely pass through easily. 19. The strip would bend, because the two metals will expand at different rates. 20. The glass is also expanding, which would cause it to look like the liquid is expanding l ...
Experiment 1 - 8. Form of Energy
... although they passed different courses. It corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. ...
... although they passed different courses. It corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. ...
Manual(Exp.1)
... corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. Since the heat and the work have been using different units even tho ...
... corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. Since the heat and the work have been using different units even tho ...
Astronomy 311: Lecture 3 - Planetary Geology • Terrestrial Planets
... ∗ Differentiation converts gpe to thermal energy. Drop a brick into a pool. As the brick sinks, friction with the water heats the pool. ∗ Radioactive isotopes in planeteismals decay and release heat - can be quite large especially when the planet was young. ∗ Process started by initial collisions/im ...
... ∗ Differentiation converts gpe to thermal energy. Drop a brick into a pool. As the brick sinks, friction with the water heats the pool. ∗ Radioactive isotopes in planeteismals decay and release heat - can be quite large especially when the planet was young. ∗ Process started by initial collisions/im ...
25 7. PASSIVE CLIMATE CONTROL FOR CULTURAL
... beneficial because it achieves more stable environments, achieves no overall cooling in an over-heated climate, nor net heating in an under-heated one. Humidity variations are also generally smaller inside buildings than outside on the same day. The rate at which interior humidities respond to the a ...
... beneficial because it achieves more stable environments, achieves no overall cooling in an over-heated climate, nor net heating in an under-heated one. Humidity variations are also generally smaller inside buildings than outside on the same day. The rate at which interior humidities respond to the a ...
Thermodynamics Problem Set - smhs
... 12. A bomb calorimeter is used to determine the specific heat of a metal. A 75.00-gram sample of the metal is heated to a temperature of 93.0oC, then quickly dropped into 125.0 grams of cold water (initial temperature is 10.0oC). If the final temperature of the water-metal mixture is 22.0oC, what is ...
... 12. A bomb calorimeter is used to determine the specific heat of a metal. A 75.00-gram sample of the metal is heated to a temperature of 93.0oC, then quickly dropped into 125.0 grams of cold water (initial temperature is 10.0oC). If the final temperature of the water-metal mixture is 22.0oC, what is ...
CHAPTER 14: Heat Answers to Questions 1. The work goes
... heating process will be interrupted. Heating will be less efficient and less uniform if the convective currents are prevented from circulating. 16. A ceiling fan makes more of a “breeze” when it is set to blow the air down (usually called the “forward” direction by fan manufacturers). This is the se ...
... heating process will be interrupted. Heating will be less efficient and less uniform if the convective currents are prevented from circulating. 16. A ceiling fan makes more of a “breeze” when it is set to blow the air down (usually called the “forward” direction by fan manufacturers). This is the se ...
heat
... Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. Calculate (a) the ...
... Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. Calculate (a) the ...
heat
... Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. Calculate (a) the ...
... Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. Calculate (a) the ...
Period 4 Activity Sheet: Transfer of Thermal Energy
... steel balls will fall off of a metal rod when it is heated. Prediction: _________________________________________ Answer: ___________________________________________ 2) What are the necessary conditions for heat transfer via conduction between two objects? c) Thermal Conductivity Your instructor wil ...
... steel balls will fall off of a metal rod when it is heated. Prediction: _________________________________________ Answer: ___________________________________________ 2) What are the necessary conditions for heat transfer via conduction between two objects? c) Thermal Conductivity Your instructor wil ...
entropy - Helios
... Q = nCPDT, CP = CV + R = (7/2) R Q = (3)(7/2)R(100) = 8725 J # 1 for heat, energy, into translation, rotation and work ...
... Q = nCPDT, CP = CV + R = (7/2) R Q = (3)(7/2)R(100) = 8725 J # 1 for heat, energy, into translation, rotation and work ...
Review Part 2
... a) Thermal energy is transferred form the water to the cup until they are both 45*C. b) Thermal energy is transferred from the cup to the water until the y are both 45*C. c) Thermal energy is transferred from the cup to the water until the cup is at 60*C and the water is at 50*C. d) No thermal energ ...
... a) Thermal energy is transferred form the water to the cup until they are both 45*C. b) Thermal energy is transferred from the cup to the water until the y are both 45*C. c) Thermal energy is transferred from the cup to the water until the cup is at 60*C and the water is at 50*C. d) No thermal energ ...
Heat Lost Heat Gained problems The heat lost by one substance in
... Heat Lost Heat Gained problems The heat lost by one substance in a system is gained by another in the system when there is a difference in temperature between the substances. There is also a heat transfer between the system and its surroundings if they are at different temperatures. ...
... Heat Lost Heat Gained problems The heat lost by one substance in a system is gained by another in the system when there is a difference in temperature between the substances. There is also a heat transfer between the system and its surroundings if they are at different temperatures. ...