ME 313 CH 7 Example Solutions
... 10) A flat plate of width 1 m is maintained at a uniform surface temperature of Ts = 150oC by using independently controlled, heat generating rectangular modules of thickness a = 10 mm and length b = 50 mm. Each module is insulated from its neighbors, as well as on its back side. Atmospheric air at ...
... 10) A flat plate of width 1 m is maintained at a uniform surface temperature of Ts = 150oC by using independently controlled, heat generating rectangular modules of thickness a = 10 mm and length b = 50 mm. Each module is insulated from its neighbors, as well as on its back side. Atmospheric air at ...
Unit B—Energy Flow in Technological Systems
... 2. The total energy in a system, including heat, remains constant. When the ball hits the floor, all the kinetic energy is not converted to kinetic energy as the ball moves back up. Some energy is lost as heat during the collision with the floor. 3. A device or a machine can be considered a perpetua ...
... 2. The total energy in a system, including heat, remains constant. When the ball hits the floor, all the kinetic energy is not converted to kinetic energy as the ball moves back up. Some energy is lost as heat during the collision with the floor. 3. A device or a machine can be considered a perpetua ...
Hess's Law
... (THERMOCHEMISTRY OR THERMODYNAMICS) Chemical Reactivity What drives chemical reactions? THERMODYNAMICS How do they occur? KINETICS ...
... (THERMOCHEMISTRY OR THERMODYNAMICS) Chemical Reactivity What drives chemical reactions? THERMODYNAMICS How do they occur? KINETICS ...
Energy Savings - Boston Heating Supply
... • Temperature and pressure relief valve is included with all models • These Rheem water heaters meet or exceed the National Appliance Energy Conservation Act (NAECA) requirements * Ask your dealer for complete warranty information. In keeping with its policy of continuous progress and product improv ...
... • Temperature and pressure relief valve is included with all models • These Rheem water heaters meet or exceed the National Appliance Energy Conservation Act (NAECA) requirements * Ask your dealer for complete warranty information. In keeping with its policy of continuous progress and product improv ...
1 - mrfiust
... a. Describe the differences in the average motion of the water molecules at point A and at point B shortly after the hot plate is turned on. b. The water is heated until a thermometer placed in the center of the container reaches 100°C. Compare the average motion of the water molecules at points A a ...
... a. Describe the differences in the average motion of the water molecules at point A and at point B shortly after the hot plate is turned on. b. The water is heated until a thermometer placed in the center of the container reaches 100°C. Compare the average motion of the water molecules at points A a ...
Thermochemistry - Harrison High School
... In calculating the total energy required to heat a chunk of ice from a temperature in the area of Part A all the way to a temperature in the area of Part E requires five different steps. The energy from each step (given in kJ) is then added up to give the total energy involved in this (Physical or C ...
... In calculating the total energy required to heat a chunk of ice from a temperature in the area of Part A all the way to a temperature in the area of Part E requires five different steps. The energy from each step (given in kJ) is then added up to give the total energy involved in this (Physical or C ...
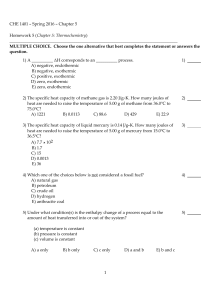
CHE 1401 - Spring 2016 - Chapter 5 Homework 5 (Chapter 5
... E) 1.44 39) Which one of the following conditions would always result in an increase in the internal energy of a system? A) The system gains heat and has work done on it by the surroundings. B) The system gains heat and does work on the surroundings. C) The system loses heat and does work on the sur ...
... E) 1.44 39) Which one of the following conditions would always result in an increase in the internal energy of a system? A) The system gains heat and has work done on it by the surroundings. B) The system gains heat and does work on the surroundings. C) The system loses heat and does work on the sur ...
CHE 1401 - Summer 2012 - Chapter 5 Homework 5 (Chapter 5
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
CHE 1401 - Fall 2016 - Chapter 5 Homework 5 (Chapter 5
... __________ and has a __________ ΔH at constant pressure. A) endothermic, positive B) endothermic, negative C) exothermic, negative D) exothermic, positive E) exothermic, neutral ...
... __________ and has a __________ ΔH at constant pressure. A) endothermic, positive B) endothermic, negative C) exothermic, negative D) exothermic, positive E) exothermic, neutral ...
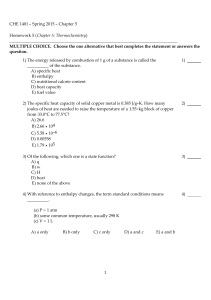
CHE 1401 - Fall 2015 - Chapter 5 Homework 5 (Chapter 5
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat required to raise the temperature of 1 lb of water by 1°F. There are _________ ...
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat required to raise the temperature of 1 lb of water by 1°F. There are _________ ...
CHE 1401 - Spring 2015 - Chapter 5 Homework 5 (Chapter 5
... B) The system gains heat and has work done on it by the surroundings. C) The system loses heat and does work on the surroundings. D) The system loses heat and has work done on it by the surroundings. E) None of the above is correct. ...
... B) The system gains heat and has work done on it by the surroundings. C) The system loses heat and does work on the surroundings. D) The system loses heat and has work done on it by the surroundings. E) None of the above is correct. ...
Key terms in low-temperature insulation
... Convection makes a considerable contribution towards improving the heat transfer coefficient. The faster the ambient air flows,the more heat is transported. In practice, it is therefore essential to ensure that pipes and ducts do not lie too close to each other or at an insufficient distance from wa ...
... Convection makes a considerable contribution towards improving the heat transfer coefficient. The faster the ambient air flows,the more heat is transported. In practice, it is therefore essential to ensure that pipes and ducts do not lie too close to each other or at an insufficient distance from wa ...
Heat and Mass Transfer B4- OGILVIE
... Different types of crystallizers have developed through the years with different shapes and sizes and different ways of inducing supersaturation like an Agitated Batch Crystallizer, Krystal Crystallizer, and Vacuum Crystallizer. But in this paper a scraped surface crystallizer will be discussed spec ...
... Different types of crystallizers have developed through the years with different shapes and sizes and different ways of inducing supersaturation like an Agitated Batch Crystallizer, Krystal Crystallizer, and Vacuum Crystallizer. But in this paper a scraped surface crystallizer will be discussed spec ...
Meltdown: Heat Conduction in Different Materials
... 5. Take a piece of straw and tape it tightly to the outside edge of each of the black blocks. Try to put the straw in the same place on each block. 6. Put the blocks down on a flat surface next to their labels—make sure you don’t mix them up! Place a black rubber O-ring in the middle of each block. ...
... 5. Take a piece of straw and tape it tightly to the outside edge of each of the black blocks. Try to put the straw in the same place on each block. 6. Put the blocks down on a flat surface next to their labels—make sure you don’t mix them up! Place a black rubber O-ring in the middle of each block. ...
1 - UCSB C.L.A.S.
... surroundings? (cwater = 4.18 J/ºC g) 4. Consider the reaction: CaCl2(s) → Ca2+(aq) + 2Cl-(aq) ΔH = -81.5 kJ If 20.0 g of calcium chloride are dissolved in 150 mL of water at 25.0 C, what will be the final temperature of the solution assuming no heat loss to the surroundings? 5. Define the following ...
... surroundings? (cwater = 4.18 J/ºC g) 4. Consider the reaction: CaCl2(s) → Ca2+(aq) + 2Cl-(aq) ΔH = -81.5 kJ If 20.0 g of calcium chloride are dissolved in 150 mL of water at 25.0 C, what will be the final temperature of the solution assuming no heat loss to the surroundings? 5. Define the following ...
Worksheet answers
... H°reaction = [2(-393.5 KJ) + 2(-285.8 KJ)] – [2(-52.4 KJ)] = -1253.8 KJ In the U.S.A., each person uses > 105 kWh of energy per year. Most comes from the combustion of fossil fuels (e.g. coal, methane, petroleum); global air temperature has risen 0.6 oC in the past 100 years. These cannot be replen ...
... H°reaction = [2(-393.5 KJ) + 2(-285.8 KJ)] – [2(-52.4 KJ)] = -1253.8 KJ In the U.S.A., each person uses > 105 kWh of energy per year. Most comes from the combustion of fossil fuels (e.g. coal, methane, petroleum); global air temperature has risen 0.6 oC in the past 100 years. These cannot be replen ...
CHEMICAL THERMODYNAMICS ANSWERS energy = anything that
... H°reaction = [2(-393.5 KJ) + 2(-285.8 KJ)] – [2(-52.4 KJ)] = -1253.8 KJ In the U.S.A., each person uses > 105 kWh of energy per year. Most comes from the combustion of fossil fuels (e.g. coal, methane, petroleum); global air temperature has risen 0.6 oC in the past 100 years. These cannot be replen ...
... H°reaction = [2(-393.5 KJ) + 2(-285.8 KJ)] – [2(-52.4 KJ)] = -1253.8 KJ In the U.S.A., each person uses > 105 kWh of energy per year. Most comes from the combustion of fossil fuels (e.g. coal, methane, petroleum); global air temperature has risen 0.6 oC in the past 100 years. These cannot be replen ...
Second Progress Report.pdf
... Several investigations and studies have been completed concerning the subject of materials for TE devices and recovering waste heat as a green energy source. Thermoelectric technology is used in countless applications to power small electronics, or harness enough energy from large heat producing sou ...
... Several investigations and studies have been completed concerning the subject of materials for TE devices and recovering waste heat as a green energy source. Thermoelectric technology is used in countless applications to power small electronics, or harness enough energy from large heat producing sou ...
Evaluation of Thermal Energy Storage Materials for Solar
... Of the above methods, sensible and latent heat storage systems are in use, chemical energy storage systems are being proposed for use in the future for medium and high temperature applications. The specific application for which a thermal storage system is to be used determines the method to be adop ...
... Of the above methods, sensible and latent heat storage systems are in use, chemical energy storage systems are being proposed for use in the future for medium and high temperature applications. The specific application for which a thermal storage system is to be used determines the method to be adop ...
WATER AS PHASE CHANGE MATERIAL IN HEAT STORAGE
... Solar radiation is the most abundant source of useful energy on the Earth. In spite of that, it is not used in large extent for covering energy demands of the human population. The diurnal and seasonal cycle of the solar radiation access may be one of reasons, even though the equally random source, ...
... Solar radiation is the most abundant source of useful energy on the Earth. In spite of that, it is not used in large extent for covering energy demands of the human population. The diurnal and seasonal cycle of the solar radiation access may be one of reasons, even though the equally random source, ...
Heat - Ms. Bergman`s Classes at DCIS Montbello
... 1. What is the difference between heat and temperature? a) Temperature measures the motion of molecules, and heat is the energy of that motion b) Temperature is measured by a thermometer, and heat is measured by a barometer c) Heat is measured in calories, and temperature is measured in joules d) He ...
... 1. What is the difference between heat and temperature? a) Temperature measures the motion of molecules, and heat is the energy of that motion b) Temperature is measured by a thermometer, and heat is measured by a barometer c) Heat is measured in calories, and temperature is measured in joules d) He ...
q - webhosting.au.edu
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, pressure, volume, temperature ...
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, pressure, volume, temperature ...
P in - XAMK Moodle
... position of the solid mass cannot change suddenly. They are subjects to delays which are dependent on the magnitude of the capacity and the resistance to the inflow of material or energy. Heater process: Electric hot water boiler has electric power Pin (w). We assume that the metal parts of the syst ...
... position of the solid mass cannot change suddenly. They are subjects to delays which are dependent on the magnitude of the capacity and the resistance to the inflow of material or energy. Heater process: Electric hot water boiler has electric power Pin (w). We assume that the metal parts of the syst ...
File - Prairie Science
... heat needed to raise the temperature of 1 g of pure water 1 oC. Used except when referring to food a Calorie, written with a capital C, always refers to the energy in food 1 Calorie = 1 kilocalorie = 1000 cal. ...
... heat needed to raise the temperature of 1 g of pure water 1 oC. Used except when referring to food a Calorie, written with a capital C, always refers to the energy in food 1 Calorie = 1 kilocalorie = 1000 cal. ...