Nuclear Chemistry 1997 D
... in part (a) is slightly less than that of the original a(234,94) Pu because of the Binding Energy in order for fission to occur, there must be a release of neutrons, forming energy. This energy is known as the “mass defect” where mass is converted into energy as shown in Einstein’s equation ...
... in part (a) is slightly less than that of the original a(234,94) Pu because of the Binding Energy in order for fission to occur, there must be a release of neutrons, forming energy. This energy is known as the “mass defect” where mass is converted into energy as shown in Einstein’s equation ...
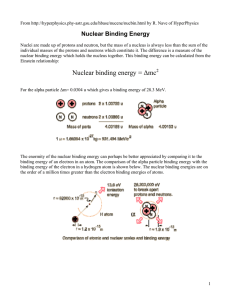
Nuclear binding energy = Δmc2 - University of Toronto Physics
... means that either the breakup of heavier nuclei (fission) or the combining of lighter nuclei (fusion) will yield nuclei which are more tightly bound (less mass per nucleon). The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. W ...
... means that either the breakup of heavier nuclei (fission) or the combining of lighter nuclei (fusion) will yield nuclei which are more tightly bound (less mass per nucleon). The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. W ...
A.6 Review questions key
... 12. Since stars and galaxies are moving towards/away from each other this proves that the universe is shrinking/expanding. One effect that helps to explain this theory is called the violet/red shift and is further evidence for the ____big____ _____bang____ theory. ...
... 12. Since stars and galaxies are moving towards/away from each other this proves that the universe is shrinking/expanding. One effect that helps to explain this theory is called the violet/red shift and is further evidence for the ____big____ _____bang____ theory. ...
Solution to Problem Set 1 1. The total number of nucleons in one
... to initiate the nuclear burning. [2 marks] Very massive stars are dominated by radiation pressure, such radiation dominated stars are unstable to radial perturbations, and hence this provides an upper limit on the stellar mass. [2 marks] At the late evolutionary stage of the Sun, helium nuclear burn ...
... to initiate the nuclear burning. [2 marks] Very massive stars are dominated by radiation pressure, such radiation dominated stars are unstable to radial perturbations, and hence this provides an upper limit on the stellar mass. [2 marks] At the late evolutionary stage of the Sun, helium nuclear burn ...
05 shell model
... It turns out that once again the Saxon-Woods model is a reasonable guess, i.e. V (r) = − ...
... It turns out that once again the Saxon-Woods model is a reasonable guess, i.e. V (r) = − ...
12_physics_notes_ch13_nuclei
... • Binding Energy: a) It may be defined as the energy required to break a nucleus into its constituent protons and neutrons and to separate them to such a large distance that they may not interact with each ...
... • Binding Energy: a) It may be defined as the energy required to break a nucleus into its constituent protons and neutrons and to separate them to such a large distance that they may not interact with each ...
Chapter #20 Nuclear Chemistry
... less stable to a more stable state In nuclear reactions more stable nuclei can be achieved by combining nuclei (fusion) or splitting a nucleus (fission) Lighter elements typically undergo fusion, while elements heavier than iron undergo fission. ...
... less stable to a more stable state In nuclear reactions more stable nuclei can be achieved by combining nuclei (fusion) or splitting a nucleus (fission) Lighter elements typically undergo fusion, while elements heavier than iron undergo fission. ...
Document
... EB = strong nuclear force binding -surface tension binding + spin pairing +shell binding-Coulomb repulsion 1) strong nuclear force -- the more nucleons the better 2) surface tension -- the less surface/volume the better (U better than He) 3) spin pairing -- neutrons and protons have + and - spins, p ...
... EB = strong nuclear force binding -surface tension binding + spin pairing +shell binding-Coulomb repulsion 1) strong nuclear force -- the more nucleons the better 2) surface tension -- the less surface/volume the better (U better than He) 3) spin pairing -- neutrons and protons have + and - spins, p ...
3 main types of particle
... Name the 2 key items involved What is the relationship between the rate of ...
... Name the 2 key items involved What is the relationship between the rate of ...
entc 4390 medical imaging
... Beta particles are electrons. However, the atom does not emit its atomic electrons. Beta electrons are emitted by a nucleus along with a neutral weakly interacting particle called the neutrino when one of the neutrons in the nucleus decays. Free neutrons are unstable - they decay. Sometimes in atoms ...
... Beta particles are electrons. However, the atom does not emit its atomic electrons. Beta electrons are emitted by a nucleus along with a neutral weakly interacting particle called the neutrino when one of the neutrons in the nucleus decays. Free neutrons are unstable - they decay. Sometimes in atoms ...
strong force
... nucleus plus the masses of Z electrons The atomic masses of different isotopes are different The periodic table contains an average value of the atomic mass for each element based on the natural abundance of each isotope The value listed in the periodic table is the mass in grams of 1 mole [Avogadro ...
... nucleus plus the masses of Z electrons The atomic masses of different isotopes are different The periodic table contains an average value of the atomic mass for each element based on the natural abundance of each isotope The value listed in the periodic table is the mass in grams of 1 mole [Avogadro ...
atoms, three states of matter.
... elements, of which there are more than 100. If, in theory, we cut a block of iron into smaller and smaller pieces, we would finally end up with the smallest piece possible that still has all the characteristics of the iron element. That smallest piece is called an iron atom. An atom is very, very sm ...
... elements, of which there are more than 100. If, in theory, we cut a block of iron into smaller and smaller pieces, we would finally end up with the smallest piece possible that still has all the characteristics of the iron element. That smallest piece is called an iron atom. An atom is very, very sm ...
1 slide per page() - Wayne State University Physics and Astronomy
... Neutrons are emitted when 235U undergoes fission These neutrons are then available to trigger fission in other nuclei This process is called a chain reaction If uncontrolled, a violent explosion can occur The principle behind the nuclear bomb, where 1 g of U can release energy equal to about 300 ...
... Neutrons are emitted when 235U undergoes fission These neutrons are then available to trigger fission in other nuclei This process is called a chain reaction If uncontrolled, a violent explosion can occur The principle behind the nuclear bomb, where 1 g of U can release energy equal to about 300 ...
Lecture 16: Iron Core Collapse, Neutron Stars
... As the mass of the nucleus increases above some value, the strong force has greater difficulty binding the large collection of neutrons and protons and the electrical repulsion becomes important. The nucleus can fission. ...
... As the mass of the nucleus increases above some value, the strong force has greater difficulty binding the large collection of neutrons and protons and the electrical repulsion becomes important. The nucleus can fission. ...
Neutron Spectroscopic Factors of 34Ar and 46Ar from transfer
... have been extensively used to understand single particle properties of nuclear structure to astrophysical network calculations. A recent analysis of ground state neutron spectroscopic factors from Z=3-24, using the conventional transfer reaction analysis, indicates that spectroscopic factors from (p ...
... have been extensively used to understand single particle properties of nuclear structure to astrophysical network calculations. A recent analysis of ground state neutron spectroscopic factors from Z=3-24, using the conventional transfer reaction analysis, indicates that spectroscopic factors from (p ...
physics 30 Matter assignment 4 - ND
... 3.0 x 10-2 T respectively. The speed of the electron, expressed in scientific notation, is b x 10w m/s. The value of w is __________. (Write the value of w, do not use significant digits. ) 3. A beam of charged particles moves undeflected through a region of perpendicular electric and magnetic field ...
... 3.0 x 10-2 T respectively. The speed of the electron, expressed in scientific notation, is b x 10w m/s. The value of w is __________. (Write the value of w, do not use significant digits. ) 3. A beam of charged particles moves undeflected through a region of perpendicular electric and magnetic field ...
Period 10 Activity Solutions: Nuclear Reactions
... e) How much energy is given off per nucleon if two deuterium nuclei fuse to form one helium nucleus? 7 MeV – 1 MeV = 6 MeV per nucleon f) How much energy is given off per helium nucleus formed? Since there are 4 nucleons in 42 He , the total energy released is 4 nucleons times 6 MeV/nucleon = 24 MeV ...
... e) How much energy is given off per nucleon if two deuterium nuclei fuse to form one helium nucleus? 7 MeV – 1 MeV = 6 MeV per nucleon f) How much energy is given off per helium nucleus formed? Since there are 4 nucleons in 42 He , the total energy released is 4 nucleons times 6 MeV/nucleon = 24 MeV ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.