The Magic of Star Dust - Exploring Exotic Nuclei
... quantum mechanics. We can never know where a nucleon is at any given time, only where it is likely to be. (The same is true for electrons in atoms.) Finally, there is not a sharp edge to a nucleus; the density is mostly constant, but as one moves out from the nucleus, it decreases smoothly to zero. ...
... quantum mechanics. We can never know where a nucleon is at any given time, only where it is likely to be. (The same is true for electrons in atoms.) Finally, there is not a sharp edge to a nucleus; the density is mostly constant, but as one moves out from the nucleus, it decreases smoothly to zero. ...
Chapter 11 Notes
... The element’s chemical symbol is used. A subscript to the left of the chemical symbol represents the atom’s atomic number Z. A superscript to the left of the chemical symbol represents the atom’s atomic mass A. ...
... The element’s chemical symbol is used. A subscript to the left of the chemical symbol represents the atom’s atomic number Z. A superscript to the left of the chemical symbol represents the atom’s atomic mass A. ...
Atomic Structure, the Periodic Table, and Nuclear Radiation
... and combines it with a proton to form a neutron. ...
... and combines it with a proton to form a neutron. ...
Higher Homework Assignments – 2013 All these homework
... Include details of any data you would need and calculations you may make. Include a discussion of any likely sources of error. ...
... Include details of any data you would need and calculations you may make. Include a discussion of any likely sources of error. ...
Electric Charge, Forces and Fields Review Worksheet (Honors)
... mass, find the Q/m ratio required for the moon to follow its present orbit of 3.84 x 10 8 m radius with its period of 27.3 days. The Earth’s mass is 5.98 x 1024 kg, and the moon’s mass is 7.3 x 1022 kg. 7. Three charges of +q, +q, and –q are at the vertices of an equilateral triangle x m per side. F ...
... mass, find the Q/m ratio required for the moon to follow its present orbit of 3.84 x 10 8 m radius with its period of 27.3 days. The Earth’s mass is 5.98 x 1024 kg, and the moon’s mass is 7.3 x 1022 kg. 7. Three charges of +q, +q, and –q are at the vertices of an equilateral triangle x m per side. F ...
Nuclear Chemistry - Moorpark College
... March 11, 2011, the Tōhoku 9.0 earthquake near the island of Honshu and following (43–49 ft) tsunami led to multiple meltdowns at Fukushima I nuclear power facility. Reactors on Units 1, 2, and 3 were operating and underwent an automatic shutdown when the earthquake struck. Stopping the normal sourc ...
... March 11, 2011, the Tōhoku 9.0 earthquake near the island of Honshu and following (43–49 ft) tsunami led to multiple meltdowns at Fukushima I nuclear power facility. Reactors on Units 1, 2, and 3 were operating and underwent an automatic shutdown when the earthquake struck. Stopping the normal sourc ...
Chapter 29 Solutions
... The proton is fired from a distance much greater than the nuclear diameter, so ri ≈ ∞ and Ui ≈ 0 J. Because the nucleus is so small, a proton that is even a few atoms away is, for all practical purposes, at infinity. As the proton approaches the nucleus, it is slowed by the repulsive electric force. ...
... The proton is fired from a distance much greater than the nuclear diameter, so ri ≈ ∞ and Ui ≈ 0 J. Because the nucleus is so small, a proton that is even a few atoms away is, for all practical purposes, at infinity. As the proton approaches the nucleus, it is slowed by the repulsive electric force. ...
Chapter 37
... stability have a neutron-to-proton ratio smaller than that needed for a stable nucleus. – These nuclides tend to decay by positron emission or electron capture because it increases the neutron to proton ratio. pn more protons than needed for stability ...
... stability have a neutron-to-proton ratio smaller than that needed for a stable nucleus. – These nuclides tend to decay by positron emission or electron capture because it increases the neutron to proton ratio. pn more protons than needed for stability ...
The Sun: Our Star (Chapter 14) The source of the Sun`s energy has
... geology needed 100s of millions of years to form, and physicists argued that no energy source could make the Sun shine for that long. The solution was the discovery of E=mc2 and nuclear reactions. The Sun’s mass is 300,000x Earth’s and its radius is 100x Earth’s. It rotates every 25 days at the equa ...
... geology needed 100s of millions of years to form, and physicists argued that no energy source could make the Sun shine for that long. The solution was the discovery of E=mc2 and nuclear reactions. The Sun’s mass is 300,000x Earth’s and its radius is 100x Earth’s. It rotates every 25 days at the equa ...
Week 1b_2015
... To produce elements heavier than H and He, nuclear particles had to be added to their nucleus in order to increase the number of protons in the nucleus. In contrast to chemical reactions which involve sharing of electrons in different atoms and can readily ...
... To produce elements heavier than H and He, nuclear particles had to be added to their nucleus in order to increase the number of protons in the nucleus. In contrast to chemical reactions which involve sharing of electrons in different atoms and can readily ...
Abundances - Michigan State University
... Element symbol – defined by charge number C is Carbon and Z=6 ...
... Element symbol – defined by charge number C is Carbon and Z=6 ...
Solutions to exam 2 - University of Rochester
... the shock wave of a supernova explosion at the end of the stellar life cycle for a large ...
... the shock wave of a supernova explosion at the end of the stellar life cycle for a large ...
nuclear physics - rct study guide
... the observation that fission occurs in these fissionable nuclei only when the neutron has approximately 1 MeV of kinetic energy. The situation is quite different for U-235, U-233, and Pu-239. In these cases, the neutron binding energy exceeds the critical energy for fission. Thus, these nuclei may b ...
... the observation that fission occurs in these fissionable nuclei only when the neutron has approximately 1 MeV of kinetic energy. The situation is quite different for U-235, U-233, and Pu-239. In these cases, the neutron binding energy exceeds the critical energy for fission. Thus, these nuclei may b ...
Snímek 1
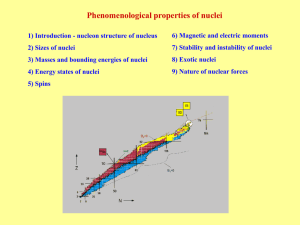
... Magic numbers – observed values of N and Z with increased stability. At 1896 H. Becquerel observed first sign of instability of nuclei – radioactivity. Instable nuclei irradiate: Alpha decay → nucleus transformation by 4He irradiation Beta decay → nucleus transformation by e-, e+ irradiation or capt ...
... Magic numbers – observed values of N and Z with increased stability. At 1896 H. Becquerel observed first sign of instability of nuclei – radioactivity. Instable nuclei irradiate: Alpha decay → nucleus transformation by 4He irradiation Beta decay → nucleus transformation by e-, e+ irradiation or capt ...
Nuclear Chemistry - Duplin County Schools
... • Isotopes of the heavier elements are stable when the ratio of neutrons to protons is about 3 to 2. ...
... • Isotopes of the heavier elements are stable when the ratio of neutrons to protons is about 3 to 2. ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.