Atomic Physics Applications
... Conservation of energy requires that the electron kinetic energy equal the maximum photon energy where we neglect the work function because it is normally so small compared to the potential energy of the electron. This yields the Duane-Hunt limit which was first found experimentally. The photon wave ...
... Conservation of energy requires that the electron kinetic energy equal the maximum photon energy where we neglect the work function because it is normally so small compared to the potential energy of the electron. This yields the Duane-Hunt limit which was first found experimentally. The photon wave ...
The title of my PDF
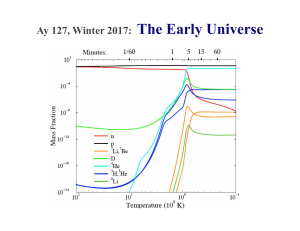
... elements are produced, we are guaranteed to have free neutrons continually resupplied to the system which recombine into heavier elements which will in turn fission again and create a feedback loop in the r-process inside the QN ejecta. This allows for continued neutron captures and “r-processing” m ...
... elements are produced, we are guaranteed to have free neutrons continually resupplied to the system which recombine into heavier elements which will in turn fission again and create a feedback loop in the r-process inside the QN ejecta. This allows for continued neutron captures and “r-processing” m ...
Modification of the Strong Nuclear Force by the
... Thus the Heisenberg Uncertainty Principle sets a fundamental limit on the precision with which these conjugate quantities are allowed to be determined. Now if we work out the quantum version of a simple mechanical harmonic oscillator—e.g., a mass on a spring—in the above respect, then due to the req ...
... Thus the Heisenberg Uncertainty Principle sets a fundamental limit on the precision with which these conjugate quantities are allowed to be determined. Now if we work out the quantum version of a simple mechanical harmonic oscillator—e.g., a mass on a spring—in the above respect, then due to the req ...
ASTRONOMY 120: GALAXIES AND THE UNIVERSE HOMEWORK
... Calculate the following for a main sequence star with T=10,000K, R=2.0R(sun), M=3.0M(sun). Assume that the proton-proton chain is the only nuclear reaction. Assume that 74% of the original mass of the star is in the form of Hydrogen, 25% in the form of Helium, and 1% in heavier elements. Assume that ...
... Calculate the following for a main sequence star with T=10,000K, R=2.0R(sun), M=3.0M(sun). Assume that the proton-proton chain is the only nuclear reaction. Assume that 74% of the original mass of the star is in the form of Hydrogen, 25% in the form of Helium, and 1% in heavier elements. Assume that ...
Introduction - Assets - Cambridge
... nuclear particles – neutrons, protons, mesons – are constructed, to the fluidlike phenomena observed when one nucleus containing many protons and neutrons collides with another. This is the subject of textbooks, monographs, and popular books on nuclear and elementary particle physics; and it is not ...
... nuclear particles – neutrons, protons, mesons – are constructed, to the fluidlike phenomena observed when one nucleus containing many protons and neutrons collides with another. This is the subject of textbooks, monographs, and popular books on nuclear and elementary particle physics; and it is not ...
Nuclear Physics Fundamental and Application Prof. H. C. Verma
... path. You know all that geometry can be done, here I am interested in finding this minimum separation here, minimum separation. So, if you write the whole equation, I do not need that, but still the equation would be, if I take this as origin and then R theta this as flexibility axis as R. So, R wil ...
... path. You know all that geometry can be done, here I am interested in finding this minimum separation here, minimum separation. So, if you write the whole equation, I do not need that, but still the equation would be, if I take this as origin and then R theta this as flexibility axis as R. So, R wil ...
PowerPoint Presentation - Neutron stars, pulsars and black
... limit and there is even reason to expect them to be close to this limit as that is what initiated the core collapse in a SNII ...
... limit and there is even reason to expect them to be close to this limit as that is what initiated the core collapse in a SNII ...
Binding Energy Powerpoint
... Consider a reaction in which the mass on the left side is less than the mass on the right side. Can this occur? Yes. Consider: ...
... Consider a reaction in which the mass on the left side is less than the mass on the right side. Can this occur? Yes. Consider: ...
Version C - UCSB Physics
... the number of electrons ejected per second and their maximum kinetic energy? A) same number of electrons ejected per second; maximum kinetic energy increases B) same number of electrons ejected per second; maximum kinetic energy does not change C) more electrons ejected per second; maximum kinetic e ...
... the number of electrons ejected per second and their maximum kinetic energy? A) same number of electrons ejected per second; maximum kinetic energy increases B) same number of electrons ejected per second; maximum kinetic energy does not change C) more electrons ejected per second; maximum kinetic e ...
7. Radioactive decay
... Recall the mass chain and Beta decay plots of Fig. 7. When studying the binding energy from the SEMF we saw that at fixed A there was a minimum in the nuclear mass for a particular value of Z. In order to reach that minimum, unstable nuclides undergo beta decay to transform excess protons in neutrons ...
... Recall the mass chain and Beta decay plots of Fig. 7. When studying the binding energy from the SEMF we saw that at fixed A there was a minimum in the nuclear mass for a particular value of Z. In order to reach that minimum, unstable nuclides undergo beta decay to transform excess protons in neutrons ...
+1/2 - WordPress.com
... Number of spin states or multiplicity: If we place an magnetically active nucleus in an external magnetic field, how many orientations it can adopt. Number of spin states is given by formula: m = 2I + 1 For example, for a nucleus with I = ½, m=2*½+1=2 So it has two spin states (or, orientations, or ...
... Number of spin states or multiplicity: If we place an magnetically active nucleus in an external magnetic field, how many orientations it can adopt. Number of spin states is given by formula: m = 2I + 1 For example, for a nucleus with I = ½, m=2*½+1=2 So it has two spin states (or, orientations, or ...
Devil physics The baddest class on campus IB Physics
... balance the atomic and mass numbers State the meaning of and difference between fission and fusion Understand that nuclear fusion takes place in the core of the stars Solve problems of fission and fusion ...
... balance the atomic and mass numbers State the meaning of and difference between fission and fusion Understand that nuclear fusion takes place in the core of the stars Solve problems of fission and fusion ...
Gilbert Ch 2 - Santa Rosa Junior College
... • Fusing of fundamental/subatomic particles (protons/neutrons) created atomic nuclei ...
... • Fusing of fundamental/subatomic particles (protons/neutrons) created atomic nuclei ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.