Name
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
Lecture 12
... splitting of the atomic energy levels appear because of the interaction of the nuclear moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. Hyperfine states that are spli ...
... splitting of the atomic energy levels appear because of the interaction of the nuclear moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. Hyperfine states that are spli ...
Relativistic and non-relativistic differential equations for the quantum
... c is the velocity of light. v g is the group velocity. m0 is the rest mass. Equation (1) can also be written as ...
... c is the velocity of light. v g is the group velocity. m0 is the rest mass. Equation (1) can also be written as ...
Electromagnetic Spectrum activity
... This states that no two electrons in any atom have the same amount of energy associated with it and therefore cannot follow the same path. Therefore considering the first energy level, n= 1 ( n is the first quantum number), contains 2 electrons (maximum) these electrons have different spins :- one c ...
... This states that no two electrons in any atom have the same amount of energy associated with it and therefore cannot follow the same path. Therefore considering the first energy level, n= 1 ( n is the first quantum number), contains 2 electrons (maximum) these electrons have different spins :- one c ...
Slide 1
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
Electromagnetic Radiation
... antenna can cause electrons in another piece of metal, such as a receiving antenna, to move up and down, creating an alternating current. b. . The current can be used to produce sound from a loudspeaker, allowing for the transmission of music, television shows, and telephone signals. ...
... antenna can cause electrons in another piece of metal, such as a receiving antenna, to move up and down, creating an alternating current. b. . The current can be used to produce sound from a loudspeaker, allowing for the transmission of music, television shows, and telephone signals. ...
10.40 Thermodynamics Fall 2003
... 1. Compute U/(RT) of a mole of diatomic ideal gas molecules treating the vibrational mode classically. Assume that the vibrational frequency is 1013 s-1, and take the reference state as E = 0 when the system is in its ground state (degeneracy of 1 and no important excited electronic states). In a fe ...
... 1. Compute U/(RT) of a mole of diatomic ideal gas molecules treating the vibrational mode classically. Assume that the vibrational frequency is 1013 s-1, and take the reference state as E = 0 when the system is in its ground state (degeneracy of 1 and no important excited electronic states). In a fe ...
Chapter 9: Intermolecular Attractions and the Properties
... PLAN: Follow the rules for allowable quantum numbers found in the text. l values can be integers from 0 to n-1; ml can be integers from -l through 0 to + l. SOLUTION: For n = 3, l = 0, 1, 2 For l = 0 ml = 0 For l = 1 ml = -1, 0, or +1 For l = 2 ml = -2, -1, 0, +1, or +2 There are 9 ml values and the ...
... PLAN: Follow the rules for allowable quantum numbers found in the text. l values can be integers from 0 to n-1; ml can be integers from -l through 0 to + l. SOLUTION: For n = 3, l = 0, 1, 2 For l = 0 ml = 0 For l = 1 ml = -1, 0, or +1 For l = 2 ml = -2, -1, 0, +1, or +2 There are 9 ml values and the ...
Wave packets Uncertainty - cranson
... structure of the electrons. As elements were built by adding one proton and one electron at a time, the electrons would find a position and shape that maximized their distance from each other (from repulsion) yet kept them as close as possible to the positive nucleus (attraction). The way they posit ...
... structure of the electrons. As elements were built by adding one proton and one electron at a time, the electrons would find a position and shape that maximized their distance from each other (from repulsion) yet kept them as close as possible to the positive nucleus (attraction). The way they posit ...
Ch 9--Linear Momentum and Collisions #1
... particle. conservation of momentum (isolated system): whenever two or more particles in an isolated system interact, the total momentum of the system remains constant. collision: an event during which two particles come close to each other and interact by means of forces elastic collision: a collisi ...
... particle. conservation of momentum (isolated system): whenever two or more particles in an isolated system interact, the total momentum of the system remains constant. collision: an event during which two particles come close to each other and interact by means of forces elastic collision: a collisi ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
Molekylfysik - Leiden Univ
... 1.3 The Schrödinger Equation From the wave-particle duality, the concepts of classical physics (CP) have to be abandoned to describe microscopic systems. The dynamics of microscopic systems will be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associat ...
... 1.3 The Schrödinger Equation From the wave-particle duality, the concepts of classical physics (CP) have to be abandoned to describe microscopic systems. The dynamics of microscopic systems will be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associat ...
1st Semester Exam Review2
... A theory can not be changed according to new data. B. A theory does not have to be repeated, because it based on only one observation. C. A theory is an explanation of things and events based on many observations. D. A theory is can never become a ...
... A theory can not be changed according to new data. B. A theory does not have to be repeated, because it based on only one observation. C. A theory is an explanation of things and events based on many observations. D. A theory is can never become a ...
Student - Davison Chemistry Website
... 1. Improved Rutherford’s work by saying electrons do not lose energy in the atoms so they will stay in orbit. 2. Stated there are definite levels in which the electrons follow set paths without gaining or losing energy (Planetary Model). 3. Each level has a certain amount of energy associated with i ...
... 1. Improved Rutherford’s work by saying electrons do not lose energy in the atoms so they will stay in orbit. 2. Stated there are definite levels in which the electrons follow set paths without gaining or losing energy (Planetary Model). 3. Each level has a certain amount of energy associated with i ...
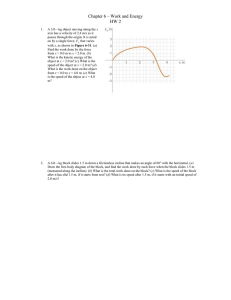
Chapter 6 HW 2
... A 6.0—kg block slides 1.5 m down a frictionless incline that makes an angle of 60° with the horizontal. (a) Draw the free-body diagram of the block, and find the work done by each force when the block slides 1.5 m (measured along the incline). (b) What is the total work done on the block? (c) What i ...
... A 6.0—kg block slides 1.5 m down a frictionless incline that makes an angle of 60° with the horizontal. (a) Draw the free-body diagram of the block, and find the work done by each force when the block slides 1.5 m (measured along the incline). (b) What is the total work done on the block? (c) What i ...