5-1-light-quantized-energy
... light could not explain a phenomenon called the photoelectric effect. O In the photoelectric effect, electrons, called photoelectrons, are emitted from a metal’s surface when light of a certain frequency, or higher than a certain frequency, shines on the ...
... light could not explain a phenomenon called the photoelectric effect. O In the photoelectric effect, electrons, called photoelectrons, are emitted from a metal’s surface when light of a certain frequency, or higher than a certain frequency, shines on the ...
Standard EPS Shell Presentation
... 14.2 Bohr model of the atom Danish physicist Neils Bohr proposed the concept of energy levels to explain the spectrum of hydrogen. When an electron moves from a higher energy level to a lower one, the atom gives up the energy difference between the two levels. The energy comes out as different ...
... 14.2 Bohr model of the atom Danish physicist Neils Bohr proposed the concept of energy levels to explain the spectrum of hydrogen. When an electron moves from a higher energy level to a lower one, the atom gives up the energy difference between the two levels. The energy comes out as different ...
stphysic - The Skeptic Tank
... What it means is that if you observer any physical laws for a given situation in your frame of reference, then an observer in a reference frame moving with a constant velocity with respect to you should also agree that those physical laws apply to that situation. >As an example, consider the conserv ...
... What it means is that if you observer any physical laws for a given situation in your frame of reference, then an observer in a reference frame moving with a constant velocity with respect to you should also agree that those physical laws apply to that situation. >As an example, consider the conserv ...
P4 revision
... In physics, units are really important. Although you are used to using mph in everyday life in physics we use m/s or metres per second. Distance is measured in metres and time in seconds. Look for tricky questions where you are given distance in km or time in minutes or hours. You might need to ...
... In physics, units are really important. Although you are used to using mph in everyday life in physics we use m/s or metres per second. Distance is measured in metres and time in seconds. Look for tricky questions where you are given distance in km or time in minutes or hours. You might need to ...
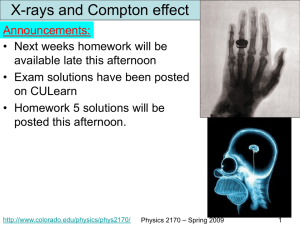
Physics 2170
... Which of the following scattered photons will have the least energy? A. A photon which continues forward (+x direction) B. A photon which emerges at a right angle (+y direction) C. A photon which comes straight back (-x direction) D. All of the scenarios above have the same energy photons If the pho ...
... Which of the following scattered photons will have the least energy? A. A photon which continues forward (+x direction) B. A photon which emerges at a right angle (+y direction) C. A photon which comes straight back (-x direction) D. All of the scenarios above have the same energy photons If the pho ...
Physics 30 - Structured Independent Learning
... It is important to note that the idea of quantization of charge does not become important until we start looking at very small objects like electrons, protons, ions, atoms, and the like. For objects with large charges involving an excess or deficit of billions and trillions of electrons, the effect ...
... It is important to note that the idea of quantization of charge does not become important until we start looking at very small objects like electrons, protons, ions, atoms, and the like. For objects with large charges involving an excess or deficit of billions and trillions of electrons, the effect ...
Chapter 4 - SchoolRack
... Rutherford’s model did not explain where electrons were – what prevented electrons from being drawn into nucleus? New model arose from experiments involving absorption and emission of light by matter ...
... Rutherford’s model did not explain where electrons were – what prevented electrons from being drawn into nucleus? New model arose from experiments involving absorption and emission of light by matter ...
Document
... The magnitude is 7 m and +ve result indicates that the motion is in the +ve direction. Answer of (b) is: When the practical moves from x1= 5 m to x2= 1 m ...
... The magnitude is 7 m and +ve result indicates that the motion is in the +ve direction. Answer of (b) is: When the practical moves from x1= 5 m to x2= 1 m ...
Essential Learning Outcomes: Physical Science - Holden R
... bands in the spectrum of electromagnetic waves and know that they all travel at the same speed in a vacuum. The student will distinguish between reflection, refraction, diffraction, Doppler effect, destructive interference and constructive interference. 9) Structure of Atoms and Matter & Chemistry ...
... bands in the spectrum of electromagnetic waves and know that they all travel at the same speed in a vacuum. The student will distinguish between reflection, refraction, diffraction, Doppler effect, destructive interference and constructive interference. 9) Structure of Atoms and Matter & Chemistry ...
view pdf - Sub-Structure of the Electron
... only the negative half wave is outside and after zero transition the „lower surface” of the positive half wave is on the outside, which is again negative from their effect. The field strength in radial direction Er of the Moebius ribbon surface is E r = E o ⋅ cos ϕ/2 ⋅ cosϕ/2 which is always mathema ...
... only the negative half wave is outside and after zero transition the „lower surface” of the positive half wave is on the outside, which is again negative from their effect. The field strength in radial direction Er of the Moebius ribbon surface is E r = E o ⋅ cos ϕ/2 ⋅ cosϕ/2 which is always mathema ...
Notes on - Paradigm Shift Now
... geometry (10). At the same time it must be pointed out that the supposedly unsatisfactory non local features of the Quantum potential Q become meaningful in the above context at the Compton scale, within which indeed we have exactly such non local effects [13]. It may be pointed out that more recent ...
... geometry (10). At the same time it must be pointed out that the supposedly unsatisfactory non local features of the Quantum potential Q become meaningful in the above context at the Compton scale, within which indeed we have exactly such non local effects [13]. It may be pointed out that more recent ...
Document
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...