File
... No other combination of statements is used as a correct response. 31 Use of the Data Booklet is relevant to this question. When the liquid N2F4 is heated, it decomposes into a single product, X. Which statements are correct? ...
... No other combination of statements is used as a correct response. 31 Use of the Data Booklet is relevant to this question. When the liquid N2F4 is heated, it decomposes into a single product, X. Which statements are correct? ...
Lecture 14
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
Intermolecular Forces
... The origin of the substantial attractive forces between nonpolar molecules was a serious problem in the early 20th century. While much was known of the strength of these forces from the Van der Waals equation of state for imperfect gases and from thermodynamic properties of liquids and solids, there ...
... The origin of the substantial attractive forces between nonpolar molecules was a serious problem in the early 20th century. While much was known of the strength of these forces from the Van der Waals equation of state for imperfect gases and from thermodynamic properties of liquids and solids, there ...
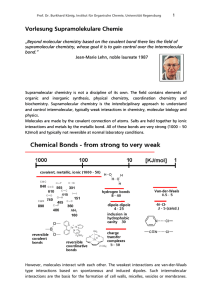
Vorlesung Supramolekulare Chemie
... Receptor molecule or guest binding site for intermolecular molecular recognition must be preorganized. The conformation and orientation of functional groups in the non-bound state should already be close to the one in the host-guest complex to minimize entropic loss. Simple non-covalent aggregates u ...
... Receptor molecule or guest binding site for intermolecular molecular recognition must be preorganized. The conformation and orientation of functional groups in the non-bound state should already be close to the one in the host-guest complex to minimize entropic loss. Simple non-covalent aggregates u ...
Chemistry - cloudfront.net
... 59. Know which type of calorimeter (coffee cup or bomb) is used when pressure or volume are held constant 60. Be able to calculate ∆G using the Gibbs Free Energy Equation (what unit does T have to be in?) 61. Know that bond formation is exothermic and bond breaking is endothermic—be able to decide w ...
... 59. Know which type of calorimeter (coffee cup or bomb) is used when pressure or volume are held constant 60. Be able to calculate ∆G using the Gibbs Free Energy Equation (what unit does T have to be in?) 61. Know that bond formation is exothermic and bond breaking is endothermic—be able to decide w ...
Journal Club - Clinical Chemistry
... Results: Performance of the Sandwich Assay. Table 2. Performance Characteristics of the Tacrolimus Sandwich Assay. a WBP1-3 = whole blood pool from transplant patients. b WPB4= whole blood pool from nontransplant patients spiked with tacrolimus powder. c Five whole blood samples from transplant pat ...
... Results: Performance of the Sandwich Assay. Table 2. Performance Characteristics of the Tacrolimus Sandwich Assay. a WBP1-3 = whole blood pool from transplant patients. b WPB4= whole blood pool from nontransplant patients spiked with tacrolimus powder. c Five whole blood samples from transplant pat ...
Raman Spectroscopy: Introductory Tutorial
... Infrared and Raman Spectra of Inorganic and Coordination Compounds : Theory and Applications in Inorganic Chemistry (Volume A) by Kazuo Nakamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds : Applications in Coordination, Organometallic, and Bioinorganic Chemistry (Volume B) by ...
... Infrared and Raman Spectra of Inorganic and Coordination Compounds : Theory and Applications in Inorganic Chemistry (Volume A) by Kazuo Nakamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds : Applications in Coordination, Organometallic, and Bioinorganic Chemistry (Volume B) by ...
Introductory Review
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
Chapter 17 Thermodynamics: Directionality of Chemical Reactions
... For the reaction ΔH° = -11,024 kJ and ΔS° = -1383.9 J/K at 298.15 K. ...
... For the reaction ΔH° = -11,024 kJ and ΔS° = -1383.9 J/K at 298.15 K. ...
The Mole
... combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
... combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
Chapter One
... combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
... combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
C:\Documents and Settings\Evan\Desktop\Chemistry\Frisch\Chem H
... a type of formula mass. The terms are sometimes used interchangeably. Step 1: Look up the mass of each element on the Formula masses are determined by following the steps in the box to the Periodic Table and round it off. right. The results are in atomic mass units (amu) Step 2: Multiply each elemen ...
... a type of formula mass. The terms are sometimes used interchangeably. Step 1: Look up the mass of each element on the Formula masses are determined by following the steps in the box to the Periodic Table and round it off. right. The results are in atomic mass units (amu) Step 2: Multiply each elemen ...
Calculations with Chemical Formulas and Equations
... – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) ...
... – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) ...
Chemical Formulas and Formula Weight Calculations
... The Mole has its origins with a hypothesis formulated by the Italian scientist Amedeo Avogadro (1776‐1856). In 1811, Avogadro pointed out that: “Gay‐Lussac has shown in an interesting Memoir (Mémoires de la Société d'Arcueil, Tome II.) that gases always unite in a very simple proportion by volume, ...
... The Mole has its origins with a hypothesis formulated by the Italian scientist Amedeo Avogadro (1776‐1856). In 1811, Avogadro pointed out that: “Gay‐Lussac has shown in an interesting Memoir (Mémoires de la Société d'Arcueil, Tome II.) that gases always unite in a very simple proportion by volume, ...
Martin Quack
... the evolution of life 1-2, the interpretation of which has been an open question for more than a century, with numerous related hypotheses, but no definitive answers. We shall briefly discuss the current status and the relation to the other two questions. The discovery of parity violation led to imp ...
... the evolution of life 1-2, the interpretation of which has been an open question for more than a century, with numerous related hypotheses, but no definitive answers. We shall briefly discuss the current status and the relation to the other two questions. The discovery of parity violation led to imp ...
Document
... subscripts by 2 or 3, we would have obtained the formulas C3H6O3 and C2H4O2, respectively. Although the ratio of carbon to hydrogen to oxygen atoms in each of these formulas is correct (1:2:1), neither is the simplest formula because the subscripts are not in the smallest possible whole-number ratio ...
... subscripts by 2 or 3, we would have obtained the formulas C3H6O3 and C2H4O2, respectively. Although the ratio of carbon to hydrogen to oxygen atoms in each of these formulas is correct (1:2:1), neither is the simplest formula because the subscripts are not in the smallest possible whole-number ratio ...
THE MOLE (a counting unit)……….Again!
... __________________________= an ionic compound containing water molecules incorporated into its solid crystal structure o In other words, a hydrate is a collection of ________________(negative ions), _____________________(positive ions), and _____________________________ ...
... __________________________= an ionic compound containing water molecules incorporated into its solid crystal structure o In other words, a hydrate is a collection of ________________(negative ions), _____________________(positive ions), and _____________________________ ...
Critical Micelar Concentration and Thermodynamic Parameters of
... The values of enthalpy variation before the CMC (curve inflection) represent the sum of molar enthalpies of micelles dilution, micelles rupture, monomers solvation, Before the CMC just the micelles dilution is registered. The variation of molar enthalpy of demicellization ( ∆ desmic H = −∆ mic H ) i ...
... The values of enthalpy variation before the CMC (curve inflection) represent the sum of molar enthalpies of micelles dilution, micelles rupture, monomers solvation, Before the CMC just the micelles dilution is registered. The variation of molar enthalpy of demicellization ( ∆ desmic H = −∆ mic H ) i ...
Full research publication
... At the same time, the ester moiety of the molecule does not form a chelate-NH depolarizing influence due to the unshared electron pairs of oxygen alkoxy, weakening the charge on the carbonyl carbon of the ester group unit and polarizing molecules DMSO strength giving the result of insufficiency This ...
... At the same time, the ester moiety of the molecule does not form a chelate-NH depolarizing influence due to the unshared electron pairs of oxygen alkoxy, weakening the charge on the carbonyl carbon of the ester group unit and polarizing molecules DMSO strength giving the result of insufficiency This ...
Week 12 - Day 1 | Day 2 | Day 3 | Lab | Skills Test
... Work the following mole calculation practice problems. Show the factor-label calculation used to work each problem. 1. A raindrop contains about 0.05 grams of water. a. How many molecules of water are in a raindrop? 2. What is the mass in grams of 0.452 mole of C6H12O6? 3. Calculate the mass in kilo ...
... Work the following mole calculation practice problems. Show the factor-label calculation used to work each problem. 1. A raindrop contains about 0.05 grams of water. a. How many molecules of water are in a raindrop? 2. What is the mass in grams of 0.452 mole of C6H12O6? 3. Calculate the mass in kilo ...
Theoretical Competition - Austrian Chemistry Olympiad
... Calculate the free reaction enthalpy ∆RGT of a mixture of 0.15 mol Borneol and 0.30 mol Isoborneol at a total pressure of 800 mbar. In which direction will this mixture react? Calculate the amounts of both substances in the equilibrium mixture, if at the beginning 7.50 g Borneol and 14.0 g iso-Borne ...
... Calculate the free reaction enthalpy ∆RGT of a mixture of 0.15 mol Borneol and 0.30 mol Isoborneol at a total pressure of 800 mbar. In which direction will this mixture react? Calculate the amounts of both substances in the equilibrium mixture, if at the beginning 7.50 g Borneol and 14.0 g iso-Borne ...
1.21 moles and formulae
... 2.16) 26.2 g of aluminium sulfate, Al2(SO4)3, was dissolved in water. Calculate the number of sulfate ions, SO42–, present in the solution formed. ...
... 2.16) 26.2 g of aluminium sulfate, Al2(SO4)3, was dissolved in water. Calculate the number of sulfate ions, SO42–, present in the solution formed. ...
Tong. ch_6.0910 - NordoniaHonorsChemistry
... units, we can find the number of moles of a constituent element if we know the number of moles of the compound. Moles of compound ...
... units, we can find the number of moles of a constituent element if we know the number of moles of the compound. Moles of compound ...
1.21. Formulae, equations and amounts of substance
... 2.16) 26.2 g of aluminium sulfate, Al2(SO4)3, was dissolved in water. Calculate the number of sulfate ions, SO42–, present in the solution formed. ...
... 2.16) 26.2 g of aluminium sulfate, Al2(SO4)3, was dissolved in water. Calculate the number of sulfate ions, SO42–, present in the solution formed. ...
Host–guest chemistry

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through noncovalent bonding. Noncovalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. There are four commonly mentioned types of non-covalent interactions: hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions.