Blog_mass - Magnetism, Bad Metals and Superconductivity
... - Second picture: In the new paper they measure 1/mass. They plot their results together with those by Sebastian, which are in a different doping range. They observe that in a given doping range 1/mass decreases with increasing doping. They extrapolate 1/m to the doping at which it would be zero and ...
... - Second picture: In the new paper they measure 1/mass. They plot their results together with those by Sebastian, which are in a different doping range. They observe that in a given doping range 1/mass decreases with increasing doping. They extrapolate 1/m to the doping at which it would be zero and ...
Lecture 17
... Classically, an electron of mass M and charge −e moving in an orbit with angular momentum L would have a magnetic moment e µ=− L 2M suggesting that in the quantum case, µ̂ = − ...
... Classically, an electron of mass M and charge −e moving in an orbit with angular momentum L would have a magnetic moment e µ=− L 2M suggesting that in the quantum case, µ̂ = − ...
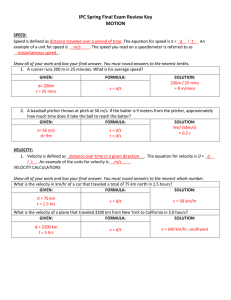
IPC Spring Final Exam Review Key MOTION
... Iron Man got into a fight with Colonel Rhodes, who was wearing the silver Iron Man suit. At the peak of their argument, both men shot lasers at one another. What was each man’s acceleration if they both flew at 5m/s in 1.5 seconds? GIVEN: FORMULA: SOLUTION: a = 3.33 m/s2 ...
... Iron Man got into a fight with Colonel Rhodes, who was wearing the silver Iron Man suit. At the peak of their argument, both men shot lasers at one another. What was each man’s acceleration if they both flew at 5m/s in 1.5 seconds? GIVEN: FORMULA: SOLUTION: a = 3.33 m/s2 ...
Atomic physics researchers need to return Bohr`s orbit
... electronic mechanical energy both of steady state orbits . According to the basic assumption, the Bohr's atomic model established and successfully explain the experimental fact that the hydrogen spectrum. However, since (1) in accordance with the traditional understanding of the necessary and suffic ...
... electronic mechanical energy both of steady state orbits . According to the basic assumption, the Bohr's atomic model established and successfully explain the experimental fact that the hydrogen spectrum. However, since (1) in accordance with the traditional understanding of the necessary and suffic ...
Quantum Gravity: The View From Particle Physics
... the Standard Model of Particle Physics or one of its possible extensions, should correctly describe the physical degrees of freedom also at the very smallest distances. The first attempt of quantizing gravity relied on canonical quantization, with the spatial metric components and their conjugate mo ...
... the Standard Model of Particle Physics or one of its possible extensions, should correctly describe the physical degrees of freedom also at the very smallest distances. The first attempt of quantizing gravity relied on canonical quantization, with the spatial metric components and their conjugate mo ...
2 THE STRUCTURE OF ATOMS
... on the type of metal used in the cathode tube, nor on the type of gas in the discharge tube. These facts suggested the possibility that the particle could be a fundamental constituent of matter. The British physicist Joseph John Thomson (1856-1940) showed that the particle possessed negative charge. ...
... on the type of metal used in the cathode tube, nor on the type of gas in the discharge tube. These facts suggested the possibility that the particle could be a fundamental constituent of matter. The British physicist Joseph John Thomson (1856-1940) showed that the particle possessed negative charge. ...
Presentation453.27
... addition to intensity. Most electronic transitions are polarized, which means that the transition dipole is not the same in all directions. The magnitude of the transition is defined as the dipole strength: ...
... addition to intensity. Most electronic transitions are polarized, which means that the transition dipole is not the same in all directions. The magnitude of the transition is defined as the dipole strength: ...
Gregory Moore - Rutgers Physics
... It is a success just as profound and notable as an experimental confirmation of a theoretical prediction. The discovery of a new and powerful invariant of four-dimensional manifolds is a vindication just as satisfying as the prediction of a new particle. ...
... It is a success just as profound and notable as an experimental confirmation of a theoretical prediction. The discovery of a new and powerful invariant of four-dimensional manifolds is a vindication just as satisfying as the prediction of a new particle. ...
F=ma by Wilczek
... bodies interact. Nowadays, of course, we know that none of that is quite true. Newton's third law states that for every action there's an equal and opposite reaction. Also, we generally assume that forces do not depend on velocity. Neither of those assumptions is quite true either; for example, they ...
... bodies interact. Nowadays, of course, we know that none of that is quite true. Newton's third law states that for every action there's an equal and opposite reaction. Also, we generally assume that forces do not depend on velocity. Neither of those assumptions is quite true either; for example, they ...
Lesson 22 questions – The Photoelectric effect and photon energy
... Well, first of all, some orbits have the same energy as other orbits, so sometimes changing orbits wouldn't emit radiation. Also, it turns out that electrons don't really move in little circular orbits. We can take a little detour to see how the Schrödinger Atom more accurately depicts what is ...
... Well, first of all, some orbits have the same energy as other orbits, so sometimes changing orbits wouldn't emit radiation. Also, it turns out that electrons don't really move in little circular orbits. We can take a little detour to see how the Schrödinger Atom more accurately depicts what is ...