Molecular Modeling Activity for Carbohydrates
... Read and highlight: The production of a disaccharide is a chemical reaction called a dehydration synthesis reaction. In such a reaction, the elements of water are removed and the glucose and fructose molecules are joined to form the disaccharide. One carbon on each participating monosaccharide is ch ...
... Read and highlight: The production of a disaccharide is a chemical reaction called a dehydration synthesis reaction. In such a reaction, the elements of water are removed and the glucose and fructose molecules are joined to form the disaccharide. One carbon on each participating monosaccharide is ch ...
Relative Reactivity of Aldehydes and Ketones: Generally
... Acidic conditions use neutral water, H2O, as the nucleophile… Neutral carbonyl and neutral water aren’t so very attracted to each other… The oxygen atom in water is electronegative and less willing to share its electron density to form a bond to the carbonyl. We must tweak the system to make them mo ...
... Acidic conditions use neutral water, H2O, as the nucleophile… Neutral carbonyl and neutral water aren’t so very attracted to each other… The oxygen atom in water is electronegative and less willing to share its electron density to form a bond to the carbonyl. We must tweak the system to make them mo ...
Chapter 8 - Power Point Presentation
... up a molecule and serves as a shorthand representation of its bonding. • Benefit: They are the most common models of molecules because they can relatively quickly allow us to show the bonding in a molecule. Drawback: They are ineffective at showing the three dimensional structure of the molecule and ...
... up a molecule and serves as a shorthand representation of its bonding. • Benefit: They are the most common models of molecules because they can relatively quickly allow us to show the bonding in a molecule. Drawback: They are ineffective at showing the three dimensional structure of the molecule and ...
How to Balance Chemical Equations
... In a chemical reaction atoms are rearranged as old chemical bonds are broken and new chemical bonds are formed. The 'law of conservation of __________' is supported as the weight doesn’t change between the mass of the reactants and the mass of the products. 2. How to balance a chemical equation In b ...
... In a chemical reaction atoms are rearranged as old chemical bonds are broken and new chemical bonds are formed. The 'law of conservation of __________' is supported as the weight doesn’t change between the mass of the reactants and the mass of the products. 2. How to balance a chemical equation In b ...
Chapter 24 Organic Chemistry
... organic bases that react with water to produce ammonia. organic acids that react with water to produce ammonia. organic bases that react with acids to form ammonium salts. organic acids that react with bases to form ammonium salts. none of these. ...
... organic bases that react with water to produce ammonia. organic acids that react with water to produce ammonia. organic bases that react with acids to form ammonium salts. organic acids that react with bases to form ammonium salts. none of these. ...
TRIPURA UNIVERSITY SURYAMANINAGAR SYLLABUS FOR B.Sc THREE-YEAR DEGREE (GENERAL AND HONOURS) COURSE
... polarity , bond polarizability, formation of σ and π bonds, localized and delocalized chemical bonds , van der Waals interaction , conjugation(resonance) , resonance energy, steric inhibition of resonance, hyperconjugation , inductive and field effects , H-bonding , dipole moment- bond moment and gr ...
... polarity , bond polarizability, formation of σ and π bonds, localized and delocalized chemical bonds , van der Waals interaction , conjugation(resonance) , resonance energy, steric inhibition of resonance, hyperconjugation , inductive and field effects , H-bonding , dipole moment- bond moment and gr ...
Document
... There are three significant reasons to study chemistry. First chemistry has important practical application in the society. The development of life saving drugs in one and a complete list would touch upon most areas of modern technology. Second chemistry is an intellectual enterprise, a way of expl ...
... There are three significant reasons to study chemistry. First chemistry has important practical application in the society. The development of life saving drugs in one and a complete list would touch upon most areas of modern technology. Second chemistry is an intellectual enterprise, a way of expl ...
Asbtracts of Talks at ICEC 2014 - Association of Chemistry Teachers
... The educational import of History and Philosophy of Science (HPS) is a growing area of science education research globally. HPS can be a useful pedagogic resource to improve science learning in different ways. Carefully prepared historical vignettes on suitable topics can help students confront thei ...
... The educational import of History and Philosophy of Science (HPS) is a growing area of science education research globally. HPS can be a useful pedagogic resource to improve science learning in different ways. Carefully prepared historical vignettes on suitable topics can help students confront thei ...
File - Garbally Chemistry
... 2. The energy supplied is not sufficient to break a C-H bond. Sufficient energy isupplied to break a Cl-Cl bond however. The energy of the radiation needs to be at least that required to homolytically spilt the chlorine molecule. 3. No molecular hydrogen produced – hence no hydrogen free radicals h ...
... 2. The energy supplied is not sufficient to break a C-H bond. Sufficient energy isupplied to break a Cl-Cl bond however. The energy of the radiation needs to be at least that required to homolytically spilt the chlorine molecule. 3. No molecular hydrogen produced – hence no hydrogen free radicals h ...
MS PowerPoint - Catalysis Eprints database
... Pauli’s exclusion principle is responsible for the fact that ordinary bulk matter is stable and occupies volume. Atoms therefore cannot be squeezed too closely together. In conductors and semi-conductors, free electrons have to share entire bulk space. Thus, their energy levels stack up, creating ba ...
... Pauli’s exclusion principle is responsible for the fact that ordinary bulk matter is stable and occupies volume. Atoms therefore cannot be squeezed too closely together. In conductors and semi-conductors, free electrons have to share entire bulk space. Thus, their energy levels stack up, creating ba ...
View flyer - Tufts University School of Engineering
... of trace acetylene (~1%) in ethylene feed streams destined for ethylene polymerization. An effective catalyst for this reaction converts all of the acetylene to ethylene without further conversion of ethylene to ethane such that there is a net increase in the amount of ethylene. Pd-Ag alloys, and mo ...
... of trace acetylene (~1%) in ethylene feed streams destined for ethylene polymerization. An effective catalyst for this reaction converts all of the acetylene to ethylene without further conversion of ethylene to ethane such that there is a net increase in the amount of ethylene. Pd-Ag alloys, and mo ...
1. All the questions are compulsory. 2. Q. N
... (b) Give any one example of these polymers and name its monomers. (c) Comment on the qualities of Shalini. 24. (a) Give a plausible explanation for each one of the following: (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid ...
... (b) Give any one example of these polymers and name its monomers. (c) Comment on the qualities of Shalini. 24. (a) Give a plausible explanation for each one of the following: (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid ...
Chemistry - CBSE Academic
... (b) Give any one example of these polymers and name its monomers. (c) Comment on the qualities of Shalini. 24. (a) Give a plausible explanation for each one of the following: (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid ...
... (b) Give any one example of these polymers and name its monomers. (c) Comment on the qualities of Shalini. 24. (a) Give a plausible explanation for each one of the following: (i) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid ...
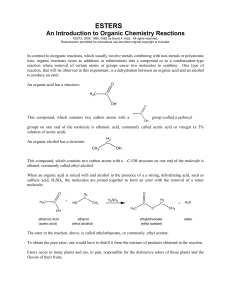
Esters - chymist.com
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
Anhydrous copper (II) sulfate: an efficient catalyst for the liquid
... the years' but is still not completely understood. Our interest in the synthesis of substituted tetrahydropyrans and deoxy ~ u g a r s ~prompted ...
... the years' but is still not completely understood. Our interest in the synthesis of substituted tetrahydropyrans and deoxy ~ u g a r s ~prompted ...
Slide 1
... Have their own phylum: Chlorobi Non-motile, except for one species Can be baccilus, coccus, or spiralus Environment must be free of oxygen Use bacteriochlorophyll c,d,& e in chlorosome vesicles on cell membrane Sulfide is reducing agent Found in black smokers @ 2500 ft. ...
... Have their own phylum: Chlorobi Non-motile, except for one species Can be baccilus, coccus, or spiralus Environment must be free of oxygen Use bacteriochlorophyll c,d,& e in chlorosome vesicles on cell membrane Sulfide is reducing agent Found in black smokers @ 2500 ft. ...