View PDF
... ____ 25. If a certain metal is placed in an ionic solution containing another metal and no reaction occurs, then the metal originally in the solution is a. a halogen. c. not on the activity series. b. higher on the activity series. d. unreactive. ____ 26. A balanced chemical equation allows one to d ...
... ____ 25. If a certain metal is placed in an ionic solution containing another metal and no reaction occurs, then the metal originally in the solution is a. a halogen. c. not on the activity series. b. higher on the activity series. d. unreactive. ____ 26. A balanced chemical equation allows one to d ...
Document
... rate. Identical strips of magnesium ribbon were dropped into different concentrations of excess hydrochloric acid and the time taken for the magnesium to completely react, recorded. A graph of the student's results is shown below. ...
... rate. Identical strips of magnesium ribbon were dropped into different concentrations of excess hydrochloric acid and the time taken for the magnesium to completely react, recorded. A graph of the student's results is shown below. ...
Minimum Learning Competencies - Ministry of Education, Ethiopia
... • Explain Energy levels, Valence electrons, and electron configuration • Write the ground state electron configuration for given elements and represent them diagrammatically • Define chemical bonding and explain why atoms form chemical bonds with other atoms • Define ionic bond, describe its formati ...
... • Explain Energy levels, Valence electrons, and electron configuration • Write the ground state electron configuration for given elements and represent them diagrammatically • Define chemical bonding and explain why atoms form chemical bonds with other atoms • Define ionic bond, describe its formati ...
as PDF - Heriot
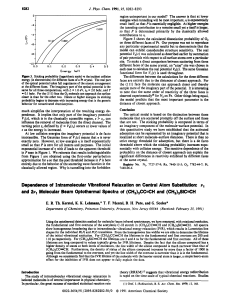
... years.1–6 Despite the fact that the main spectroscopic features have been described for a wide range of Cr (III) complexes, the nature of reactive states and photochemical relaxation mechanisms is still not known for many of them. Often with transition metal complexes electronic transitions from the ...
... years.1–6 Despite the fact that the main spectroscopic features have been described for a wide range of Cr (III) complexes, the nature of reactive states and photochemical relaxation mechanisms is still not known for many of them. Often with transition metal complexes electronic transitions from the ...
Chemistry Spell check on
... rate. Identical strips of magnesium ribbon were dropped into different concentrations of excess hydrochloric acid and the time taken for the magnesium to completely react, recorded. A graph of the student's results is shown below. ...
... rate. Identical strips of magnesium ribbon were dropped into different concentrations of excess hydrochloric acid and the time taken for the magnesium to completely react, recorded. A graph of the student's results is shown below. ...
Pyrrolidine-2-carboxylic Acid (l
... from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
... from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
A matter of Equilibrium
... The reaction N2 + 3 H2 2 NH3 has en equilibrium constant at 400 °C of 1.9 x 10-4. Suppose 1 mol N2, 0.2 mol H2 and 0.4 mol NH3 are sealed in a 1 dm3 flask at 400 °C. In which direction will the reaction proceed? ...
... The reaction N2 + 3 H2 2 NH3 has en equilibrium constant at 400 °C of 1.9 x 10-4. Suppose 1 mol N2, 0.2 mol H2 and 0.4 mol NH3 are sealed in a 1 dm3 flask at 400 °C. In which direction will the reaction proceed? ...
unit 4: chemical reaction rates
... Scientists discovered that by simply determining the mass of the substance, it was possible to count particles or atoms. A mole (mol) is the amount of a pure substance that contains the same amount of chemical units as there are atoms in exactly 12 grams of carbon, namely 12. In order to avoid confu ...
... Scientists discovered that by simply determining the mass of the substance, it was possible to count particles or atoms. A mole (mol) is the amount of a pure substance that contains the same amount of chemical units as there are atoms in exactly 12 grams of carbon, namely 12. In order to avoid confu ...
Cheat Sheet for Chemical Equilibrium
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
ď - Google Sites
... combining these three points, it was possible to assign a value to any atom the term electronegativity is used to describe the relative ability of an atom to attract a pair of bonding electrons in its valence level (energy level) electronegativity is usually assigned on a developed scale – acc ...
... combining these three points, it was possible to assign a value to any atom the term electronegativity is used to describe the relative ability of an atom to attract a pair of bonding electrons in its valence level (energy level) electronegativity is usually assigned on a developed scale – acc ...
pdfCfE Higher - Unit 3 - Pupil Booklet 2 MB
... zero but the aim is to learn from mistakes and reduce the rate to a minimum. It is essential at this stage to revise all of your National 5 calculations that were based on moles and equations. ...
... zero but the aim is to learn from mistakes and reduce the rate to a minimum. It is essential at this stage to revise all of your National 5 calculations that were based on moles and equations. ...
Nikolai N. Semenov - Nobel Lecture
... the combustion of carbon monoxide and oxygen mixtures and the combustion of liquid nitroglycol. Soon after, the theory of the limits of concentration of normal flame propagations, a phenomenon so important to safety techniques, was also forthcoming. We explained this phenomenon by describing how the ...
... the combustion of carbon monoxide and oxygen mixtures and the combustion of liquid nitroglycol. Soon after, the theory of the limits of concentration of normal flame propagations, a phenomenon so important to safety techniques, was also forthcoming. We explained this phenomenon by describing how the ...
Chapter 4
... With four valence electrons, carbon can form four covalent bonds with a variety of atoms This ability makes large, complex molecules possible In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral ...
... With four valence electrons, carbon can form four covalent bonds with a variety of atoms This ability makes large, complex molecules possible In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral ...
Slide 1
... With four valence electrons, carbon can form four covalent bonds with a variety of atoms This ability makes large, complex molecules possible In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral ...
... With four valence electrons, carbon can form four covalent bonds with a variety of atoms This ability makes large, complex molecules possible In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral ...
12602989_294 - University of Canterbury
... Summary of X-ray details: 2: C20H20Ag2Cl2O8, monoclinic, P21/c, Z = 4, wR2 (all data) = 0.1037, R1 [I>2σ(I)] 0.0388; 4: C30H30Ag2B2F8, monoclinic, P21/n, Z = 8, wR2 (all data) = 0.1349, R1 [I>2σ(I)] 0.0543; 5: C30H30Ag2Cl2O8, monoclinic, P21/n, Z = 8, wR2 (all data) = 0.1229, R1 [I>2σ(I)] 0.0500; 6: ...
... Summary of X-ray details: 2: C20H20Ag2Cl2O8, monoclinic, P21/c, Z = 4, wR2 (all data) = 0.1037, R1 [I>2σ(I)] 0.0388; 4: C30H30Ag2B2F8, monoclinic, P21/n, Z = 8, wR2 (all data) = 0.1349, R1 [I>2σ(I)] 0.0543; 5: C30H30Ag2Cl2O8, monoclinic, P21/n, Z = 8, wR2 (all data) = 0.1229, R1 [I>2σ(I)] 0.0500; 6: ...
KINETICS (chap 12)
... Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temperature or pressure change. Be able to use H (heat and temp) in le Chatelier's principle and K. Solve I.C.E. problems. Also know how to do ICE if your given amount ...
... Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temperature or pressure change. Be able to use H (heat and temp) in le Chatelier's principle and K. Solve I.C.E. problems. Also know how to do ICE if your given amount ...