Need
... Nuclear fusion requires very high temperatures, and is not yet ready for practical use. The main advantage it offers is that the products are not radioactive wastes (as with fission). 6. Nuclear reactions can be represented by equations that include symbols which represent atomic nuclei (with mas ...
... Nuclear fusion requires very high temperatures, and is not yet ready for practical use. The main advantage it offers is that the products are not radioactive wastes (as with fission). 6. Nuclear reactions can be represented by equations that include symbols which represent atomic nuclei (with mas ...
chemistry — released form
... Energy is absorbed when excitation occurs. Excitation is when an electron goes from a low energy level to a higher energy level. ...
... Energy is absorbed when excitation occurs. Excitation is when an electron goes from a low energy level to a higher energy level. ...
Atoms and Molecules
... member of our republic. You will learn to move beyond the memorization of subject matter (although it is often a useful tool) and move towards the challenge of applying, analyzing and thinking deeply. This will help you in every single subject you take henceforth! Whether or not you plan to be a sci ...
... member of our republic. You will learn to move beyond the memorization of subject matter (although it is often a useful tool) and move towards the challenge of applying, analyzing and thinking deeply. This will help you in every single subject you take henceforth! Whether or not you plan to be a sci ...
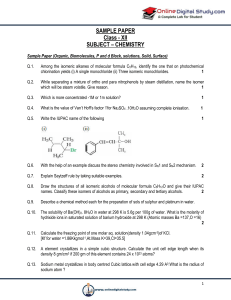
SAMPLE PAPER Class - XII SUBJECT
... Ferric hydroxide sol gets coagulated on addition of sodium chloride solution (b) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories. (c) Physical adsorption is multilayered, while chemisorption is monolayered. Q.17. Nitro group increases the reactivity of chloroben ...
... Ferric hydroxide sol gets coagulated on addition of sodium chloride solution (b) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories. (c) Physical adsorption is multilayered, while chemisorption is monolayered. Q.17. Nitro group increases the reactivity of chloroben ...
Ch. 8 Sections 8.1-8.3 Powerpoint
... •In ionic bonding the participating atoms are so different that one or more electrons are transferred to form oppositely charged ions, when then attract each other. •In covalent bonding (also called nonpolar covalent bonding) two identical atoms share electrons equally. •There are intermediate case ...
... •In ionic bonding the participating atoms are so different that one or more electrons are transferred to form oppositely charged ions, when then attract each other. •In covalent bonding (also called nonpolar covalent bonding) two identical atoms share electrons equally. •There are intermediate case ...
Things to Know to Pass the Chemistry Regents
... *<7 acidic (H+ > OH-), farther from neutral = more acidic *>7 basic (OH- . H+), farther from neutral = more basic *each move a 10x change in H+ concentration (1 is 10x stronger than 2, 1 is 100x stronger than 3) 145. All organic compounds contain C, carbon *and (usually) H, hydrogen 146. Carbon ALWA ...
... *<7 acidic (H+ > OH-), farther from neutral = more acidic *>7 basic (OH- . H+), farther from neutral = more basic *each move a 10x change in H+ concentration (1 is 10x stronger than 2, 1 is 100x stronger than 3) 145. All organic compounds contain C, carbon *and (usually) H, hydrogen 146. Carbon ALWA ...
AP Chemistry Summer Assignment 2016 revised
... the list and find other web sites that help prepare you for the coming year. We recommend that you complete as many online quizzes as possible, take detailed notes, and practice the items indicated in the packet. Completed work must be submitted by AUGUST 23th, Mail the assignments to the school add ...
... the list and find other web sites that help prepare you for the coming year. We recommend that you complete as many online quizzes as possible, take detailed notes, and practice the items indicated in the packet. Completed work must be submitted by AUGUST 23th, Mail the assignments to the school add ...
2A6
... Ag(111) and Cu(111) surfaces was investigated by means of scanning tunneling microscopy (STM) combined with density functional theory (DFT) calculations. The visible-light-induced photodissociation on metal substrates has long been thought to never occur, either because visible-light energy is much ...
... Ag(111) and Cu(111) surfaces was investigated by means of scanning tunneling microscopy (STM) combined with density functional theory (DFT) calculations. The visible-light-induced photodissociation on metal substrates has long been thought to never occur, either because visible-light energy is much ...
Solving Systems Equations Graphically
... Student Learning Assistance Center - San Antonio College ...
... Student Learning Assistance Center - San Antonio College ...
Chapter 1 Reading Guide
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
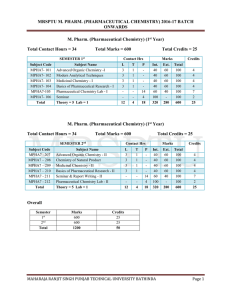
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
Note 1.1 Chemistry of Life
... Elements are made up of atoms, which are the smallest unit that maintains the chemical and physical properties of the element. An atom is made up of three different sub atomic particles; neutrons (no charge), protons (positive charge), and electrons (negative charge). Atomic number is the number of ...
... Elements are made up of atoms, which are the smallest unit that maintains the chemical and physical properties of the element. An atom is made up of three different sub atomic particles; neutrons (no charge), protons (positive charge), and electrons (negative charge). Atomic number is the number of ...
PAP Chemistry - Fall Final Review
... and reactants located in a chemical reaction? 54. Be able to balance chemical equations. a. Al4C3 + H2O CH4 + Al(OH)3 Al4C3 + 12H2O 3CH4 + 4Al(OH)3 55. Be able to recognize a synthesis reaction, a single replacement reaction, a double replacement reaction, and a decomposition reaction. 56. When ...
... and reactants located in a chemical reaction? 54. Be able to balance chemical equations. a. Al4C3 + H2O CH4 + Al(OH)3 Al4C3 + 12H2O 3CH4 + 4Al(OH)3 55. Be able to recognize a synthesis reaction, a single replacement reaction, a double replacement reaction, and a decomposition reaction. 56. When ...
Lecture 24 (Slides) October 18
... • When Main Group elements react, electrons can be transferred (usually from a metal to a nonmetal) to form ionic bonds. In other cases, pairs of electrons can be shared (usually between nonmetal atoms) to form covalent bonds. In both cases valence electrons are somehow “rearranged” when new chemica ...
... • When Main Group elements react, electrons can be transferred (usually from a metal to a nonmetal) to form ionic bonds. In other cases, pairs of electrons can be shared (usually between nonmetal atoms) to form covalent bonds. In both cases valence electrons are somehow “rearranged” when new chemica ...
Contraceptive Fact Sheet - Fertility Awareness
... women who use FAB methods are likely to get pregnant in 1 year of use. These methods can be highly effective if the instructions are followed carefully for each and every menstrual cycle. If instructions are not followed — consistently and correctly — the chance of pregnancy goes up. Fertility produ ...
... women who use FAB methods are likely to get pregnant in 1 year of use. These methods can be highly effective if the instructions are followed carefully for each and every menstrual cycle. If instructions are not followed — consistently and correctly — the chance of pregnancy goes up. Fertility produ ...
Unit 1
... Physical change: Although some extensive properties (like shape, phase, etc.) of the material change, the material itself is the same before and after the change. The change can be “undone.” ...
... Physical change: Although some extensive properties (like shape, phase, etc.) of the material change, the material itself is the same before and after the change. The change can be “undone.” ...
Chapter 1 Chemistry: Matter and Measurement
... Chemistry is concerned with matter and energy and how the two interact with each other. ...
... Chemistry is concerned with matter and energy and how the two interact with each other. ...