Compounds and Equations
... Chemical Equations use chemical formulas and physical states like solid (s), liquid (l), gases (g), and aqueous (aq) to describe a chemical reaction. These equations give us information about what is reacting, what is being formed and how much or the number of moles of each substance and even the en ...
... Chemical Equations use chemical formulas and physical states like solid (s), liquid (l), gases (g), and aqueous (aq) to describe a chemical reaction. These equations give us information about what is reacting, what is being formed and how much or the number of moles of each substance and even the en ...
Lesson 1 Magnets
... 4. When a magnetic material is close to a magnet, it becomes a magnet itself. 5. Iron is a SOFT magnetic material;it is easily magnetised but easily loses its magnetism. 6. Steel is a HARD magnetic material; it is hard to magnetise but keeps its magnetism. 7. The magnetic field around a bar magnet i ...
... 4. When a magnetic material is close to a magnet, it becomes a magnet itself. 5. Iron is a SOFT magnetic material;it is easily magnetised but easily loses its magnetism. 6. Steel is a HARD magnetic material; it is hard to magnetise but keeps its magnetism. 7. The magnetic field around a bar magnet i ...
Electromagnet
... 5.P.5A.1 Use mathematical and computational thinking to describe and predict the motion of an object (including position, direction, and speed). 5.P.5A.2 Develop and use models to explain how the amount or type of force (contact and non-contact) affects the motion of an object. 5.P.5A.3 Plan and con ...
... 5.P.5A.1 Use mathematical and computational thinking to describe and predict the motion of an object (including position, direction, and speed). 5.P.5A.2 Develop and use models to explain how the amount or type of force (contact and non-contact) affects the motion of an object. 5.P.5A.3 Plan and con ...
Chapter 8
... • The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu • 1 mole of H2O will weigh 18.02 g, therefore the molar mass of H2O is 18.02 g • 1 mole of H2O will contain 16.00 g of oxygen and 2.02 g of hydrogen ...
... • The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu • 1 mole of H2O will weigh 18.02 g, therefore the molar mass of H2O is 18.02 g • 1 mole of H2O will contain 16.00 g of oxygen and 2.02 g of hydrogen ...
Reading Guide CH 28KEYJWW
... What is magnetic domain? A magnetic domain is a small section of a ferromagnetic material in which the atoms in this section are magnetically aligned. Adjoining domains will tend to be aligned magnetically in the same way if the material is magnetized, but this tendency does not exist for domains in ...
... What is magnetic domain? A magnetic domain is a small section of a ferromagnetic material in which the atoms in this section are magnetically aligned. Adjoining domains will tend to be aligned magnetically in the same way if the material is magnetized, but this tendency does not exist for domains in ...
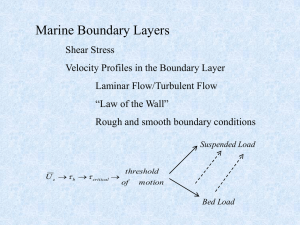
Lecture 4
... XY, YX component – assume uniform flow (flow not rotating in the mean) End up with two components: ...
... XY, YX component – assume uniform flow (flow not rotating in the mean) End up with two components: ...
POWERPOINT - Chapter 8
... Once they make it – they must determine what it is ◦ What is it's composition? ◦ What is it's chemical formula? ...
... Once they make it – they must determine what it is ◦ What is it's composition? ◦ What is it's chemical formula? ...
Chapter 7 - Faculty Web Pages
... Sorbic acid is added to food as a mold inhibitor. Its composition is 64.3% C, 7.2% H, and 28.5% O, and its molecular mass is 112 g/mol. What is its molecular formula? ...
... Sorbic acid is added to food as a mold inhibitor. Its composition is 64.3% C, 7.2% H, and 28.5% O, and its molecular mass is 112 g/mol. What is its molecular formula? ...
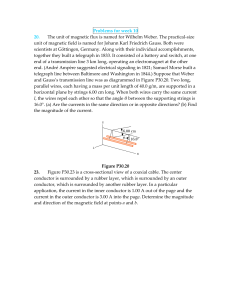
151c19
... Example 19.2: Electrons are accelerated through a potential difference of 1000 V. The electrons then enter a region where there is a magnetic field and a “crossed” electric field (E and B are perpendicular), both of which are perpendicular to the beam. What magnitude magnetic field must be applied t ...
... Example 19.2: Electrons are accelerated through a potential difference of 1000 V. The electrons then enter a region where there is a magnetic field and a “crossed” electric field (E and B are perpendicular), both of which are perpendicular to the beam. What magnitude magnetic field must be applied t ...
Coverage - Smart Science
... Recognise magnetism as a property and know some magnetic and non-magnetic materials. Know that magnets come with two poles – north and south. Describe simple interactions of magnets and correctly use the terms apply, repel. MOST students should (levels 5–6): Understand the difference between ...
... Recognise magnetism as a property and know some magnetic and non-magnetic materials. Know that magnets come with two poles – north and south. Describe simple interactions of magnets and correctly use the terms apply, repel. MOST students should (levels 5–6): Understand the difference between ...
chapter32.4 - Colorado Mesa University
... Notice: z is distance measured from the center of dipole. z >> s ...
... Notice: z is distance measured from the center of dipole. z >> s ...
7 - web page for staff
... Analogy to Gauss’s law Use for magnetostatic’s problems with sufficient symmetry. Ampere’s circuital law – the integration of around any closed path is equal to the net current enclosed by that path. ...
... Analogy to Gauss’s law Use for magnetostatic’s problems with sufficient symmetry. Ampere’s circuital law – the integration of around any closed path is equal to the net current enclosed by that path. ...
Document
... B) Are the directions of the field lines in the core consistent with the Right Hand Rule? C) Are the North and South Poles correctly ...
... B) Are the directions of the field lines in the core consistent with the Right Hand Rule? C) Are the North and South Poles correctly ...