Ch. 3 9-Station Review
... A student is assigned the task of determining the number of moles of water in one mole of MgCl2 · n H2O. The student collects the data shown in the following table. Mass of empty container Initial mass of sample and container Mass of sample and container after first heating Mass of sample and contai ...
... A student is assigned the task of determining the number of moles of water in one mole of MgCl2 · n H2O. The student collects the data shown in the following table. Mass of empty container Initial mass of sample and container Mass of sample and container after first heating Mass of sample and contai ...
Camp 1 - drjosephryan.com Home Page
... Ionic compounds, also called salts, consist of both positive and negative ions When an ionic compound dissolves in water, it dissociates to aqueous ions ...
... Ionic compounds, also called salts, consist of both positive and negative ions When an ionic compound dissolves in water, it dissociates to aqueous ions ...
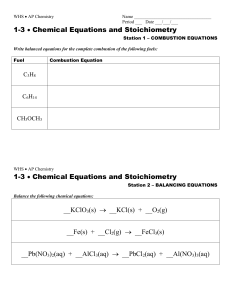
WRITING AP EQUATIONS AP equation sets are found in the
... reaction will occur. These equations need to be written in net ionic form. All spectator ions must be left out and all ions must be written in ionic form. All molecular substances and insoluble compounds must be written together (not ionized!). Know your solubility rules!!! ...
... reaction will occur. These equations need to be written in net ionic form. All spectator ions must be left out and all ions must be written in ionic form. All molecular substances and insoluble compounds must be written together (not ionized!). Know your solubility rules!!! ...
October 8: Solving Literal Equations Powerpoint
... solve for in the equation. Step 2 Identify the operations on this variable and the order in which they are applied. Step 3 Use inverse operations to undo operations and isolate the variable. ...
... solve for in the equation. Step 2 Identify the operations on this variable and the order in which they are applied. Step 3 Use inverse operations to undo operations and isolate the variable. ...
Ch.5
... Ex. If 2.00 g of a copper (II) sulfate hydrate is heated and the mass of the anhydrate is 1.28 g, what is the formula of the hydrate? ...
... Ex. If 2.00 g of a copper (II) sulfate hydrate is heated and the mass of the anhydrate is 1.28 g, what is the formula of the hydrate? ...
Chemical Reactions and Stoichiometry
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
AP Chemistry Name: Ch.2 – The Nuclear Atom Date: Period:
... 4. If the maximum amount of product possible is formed in the following reactions, what mass of the specified product would you obtain? a. 10 grams of sodium chloride is treated with excess silver nitrate: AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) How much silver chloride is precipitated? ...
... 4. If the maximum amount of product possible is formed in the following reactions, what mass of the specified product would you obtain? a. 10 grams of sodium chloride is treated with excess silver nitrate: AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) How much silver chloride is precipitated? ...
Theoretical Competition - Austrian Chemistry Olympiad
... Calculate the free reaction enthalpy ∆RGT of a mixture of 0.15 mol Borneol and 0.30 mol Isoborneol at a total pressure of 800 mbar. In which direction will this mixture react? Calculate the amounts of both substances in the equilibrium mixture, if at the beginning 7.50 g Borneol and 14.0 g iso-Borne ...
... Calculate the free reaction enthalpy ∆RGT of a mixture of 0.15 mol Borneol and 0.30 mol Isoborneol at a total pressure of 800 mbar. In which direction will this mixture react? Calculate the amounts of both substances in the equilibrium mixture, if at the beginning 7.50 g Borneol and 14.0 g iso-Borne ...
accelerated-geometry-algebra-ii-pre-requisite-packet
... 2. To solve a system by substitution, solve one equation for a single variable, then substitute the expression into the second equation and solve. Solve for the second. 3. To solve a system by elimination, put both equations in standard form Ax+By=C. Multiply one or both equations so that the coeffi ...
... 2. To solve a system by substitution, solve one equation for a single variable, then substitute the expression into the second equation and solve. Solve for the second. 3. To solve a system by elimination, put both equations in standard form Ax+By=C. Multiply one or both equations so that the coeffi ...
Lesson 4
... Now substitute 9 for x in either equation to find the value of y. First equation Simplify. Subtract 18 from each side. Simplify. Answer: The solution is (9, 5). ...
... Now substitute 9 for x in either equation to find the value of y. First equation Simplify. Subtract 18 from each side. Simplify. Answer: The solution is (9, 5). ...
Solving One-Step Equations by Adding
... solution set is the set of all solutions. Finding the solutions of an equation is also called solving the equation. ...
... solution set is the set of all solutions. Finding the solutions of an equation is also called solving the equation. ...
solution
... are developing vehicles that run on hydrogen. These cars are environmentally friendly because their only emission is water vapor. One way to obtain hydrogen for fuel is to use an emission-free energy source such as wind power to split hydrogen from water. What mass of hydrogen (in grams) does 1.00 g ...
... are developing vehicles that run on hydrogen. These cars are environmentally friendly because their only emission is water vapor. One way to obtain hydrogen for fuel is to use an emission-free energy source such as wind power to split hydrogen from water. What mass of hydrogen (in grams) does 1.00 g ...
2015 AP Chemistry Summer Assignment
... 42. Balance each of the following chemical equations a) KO2(s) + H2O(l) → KOH(aq) + O2(g) + H2O2(aq) b) Fe2O3(s) + HNO3(aq) → Fe(NO3)3(aq) + H2O(l) c) NH3(g) + O2(g) → NO(g) + H2O(g) d) PCl5(l) + H2O(l) → H3PO4(aq) + HCl(g) e) CaO(s) + C(s) → CaC2(s) + CO2(g) f) MoS2(s) + O2(g) → MoO3(s) + SO2(g) g) ...
... 42. Balance each of the following chemical equations a) KO2(s) + H2O(l) → KOH(aq) + O2(g) + H2O2(aq) b) Fe2O3(s) + HNO3(aq) → Fe(NO3)3(aq) + H2O(l) c) NH3(g) + O2(g) → NO(g) + H2O(g) d) PCl5(l) + H2O(l) → H3PO4(aq) + HCl(g) e) CaO(s) + C(s) → CaC2(s) + CO2(g) f) MoS2(s) + O2(g) → MoO3(s) + SO2(g) g) ...
Review Sheet for Test 3
... (g) Find the equation of the vertical line which passes through the point (17, −33). A vertical line has an equation of the form x = (a number). In this case, the number is the x-coordinate of (17, −33) — that is, 17. So the vertical line which passes through the point (17, −33) is x = 17. (h) Find ...
... (g) Find the equation of the vertical line which passes through the point (17, −33). A vertical line has an equation of the form x = (a number). In this case, the number is the x-coordinate of (17, −33) — that is, 17. So the vertical line which passes through the point (17, −33) is x = 17. (h) Find ...























