Chapter 3: Calculations with Chemical Formulas
... The compound contains 68.8% C, 5.0% H and 26.2% O by mass. What is the empirical formula? For a 100.0-g sample of benzoic acid, 68.8 g are C, 5.0 g are H, and 26.2 g are O. Using the molar masses, convert these masses to moles: 68.8g C x 1 mol C = 5.729 mol C 12.01g C 5.0g H x 1 mol H = 4.961 mol H ...
... The compound contains 68.8% C, 5.0% H and 26.2% O by mass. What is the empirical formula? For a 100.0-g sample of benzoic acid, 68.8 g are C, 5.0 g are H, and 26.2 g are O. Using the molar masses, convert these masses to moles: 68.8g C x 1 mol C = 5.729 mol C 12.01g C 5.0g H x 1 mol H = 4.961 mol H ...
Chapter
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
Structural determination of organic compounds
... • A method used to separate a solvent from a solution containing non-volatile solutes • When a solution is boiled, only the solvent vaporizes the hot vapour formed condenses to liquid again on a cold surface ...
... • A method used to separate a solvent from a solution containing non-volatile solutes • When a solution is boiled, only the solvent vaporizes the hot vapour formed condenses to liquid again on a cold surface ...
Solving equations using logs
... Rearrange this equation to get the two terms involving x on the right hand side: 3 log 2 = x log 2 − x log 6 www.mathcentre.ac.uk ...
... Rearrange this equation to get the two terms involving x on the right hand side: 3 log 2 = x log 2 − x log 6 www.mathcentre.ac.uk ...
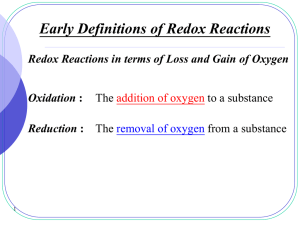
Redox reactions - SALEM-Immanuel Lutheran College
... bond (including ionic bond) between two unlike atoms is presumed to belong to the more electronegative atom. ...
... bond (including ionic bond) between two unlike atoms is presumed to belong to the more electronegative atom. ...
Chapter 3 Stoichiometry: Calculations with Chemical
... 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO ...
... 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... of each kind of atoms present in the molcule (subscript 1 is always omitted). Such a formula is called molecular formula as it represents a molecule of a substance. A molecule of water consists of two hydrogen atoms and one oxygen atom. So its molecular formula is written as H2O. Thus a molecular fo ...
... of each kind of atoms present in the molcule (subscript 1 is always omitted). Such a formula is called molecular formula as it represents a molecule of a substance. A molecule of water consists of two hydrogen atoms and one oxygen atom. So its molecular formula is written as H2O. Thus a molecular fo ...
Fill in the blanks with the appropriate word, phrase, number, or unit.
... 2. (3 points) The molecular formula of a compound with the empirical formula H5C2O and a molecular mass of 90.0 g/mole is ...
... 2. (3 points) The molecular formula of a compound with the empirical formula H5C2O and a molecular mass of 90.0 g/mole is ...
Chap18 - Bakersfield College
... Criteria for Precipitation • To determine whether an equilibrium system will go in the forward or reverse direction requires that we evaluate the reaction quotient, Qc. – To predict the direction of reaction, you compare Qc with Kc (Chapter 10 and 14) – The reaction quotient has the same form as the ...
... Criteria for Precipitation • To determine whether an equilibrium system will go in the forward or reverse direction requires that we evaluate the reaction quotient, Qc. – To predict the direction of reaction, you compare Qc with Kc (Chapter 10 and 14) – The reaction quotient has the same form as the ...
Contents and Concepts Learning Objectives
... • Exactly 0.133 mg of AgBr will dissolve in 1.00 L of water. What is the value of Ksp for AgBr? ...
... • Exactly 0.133 mg of AgBr will dissolve in 1.00 L of water. What is the value of Ksp for AgBr? ...
AP® Chemistry
... aqueous unless otherwise specified. Throughout the test the following symbols have the definitions specified unless otherwise noted. T = P = V = S = H= G= R = n = M= m= ...
... aqueous unless otherwise specified. Throughout the test the following symbols have the definitions specified unless otherwise noted. T = P = V = S = H= G= R = n = M= m= ...