11 - Edmodo
... electricity produced by the continuous flow of electrons (remember only electrons move, protons do not). In order for this flow to happen we require two things: 1) an energy source – such as a battery or electrochemical cell 2) a complete path – created by connecting wires Together this forms an ele ...
... electricity produced by the continuous flow of electrons (remember only electrons move, protons do not). In order for this flow to happen we require two things: 1) an energy source – such as a battery or electrochemical cell 2) a complete path – created by connecting wires Together this forms an ele ...
Sample Chapter - Chapter 4
... Water separates ions in a process that greatly reduces the electrostatic force of attraction between them. To see how it does this, let’s examine the water molecule closely. Water’s power as an ionizing solvent results from two features of the water molecule: the distribution of its bonding electron ...
... Water separates ions in a process that greatly reduces the electrostatic force of attraction between them. To see how it does this, let’s examine the water molecule closely. Water’s power as an ionizing solvent results from two features of the water molecule: the distribution of its bonding electron ...
N Goalby chemrevise.org 1 2.5 Transition Metals Substitution
... [Cu(H2O)6]2+ (aq) + EDTA4- (aq) [Cu (EDTA)]2- (aq) + 6H2O (l) The copper complex ion has changed from having unidentate ligands to a multidentate ligand. In this reaction there is an increase in the entropy because there are more moles of products than reactants (from 2 to 7), creating more disord ...
... [Cu(H2O)6]2+ (aq) + EDTA4- (aq) [Cu (EDTA)]2- (aq) + 6H2O (l) The copper complex ion has changed from having unidentate ligands to a multidentate ligand. In this reaction there is an increase in the entropy because there are more moles of products than reactants (from 2 to 7), creating more disord ...
Pore-Scale Controls on Reaction-Driven Fracturing - DUO
... We will now take a closer look at the pore-scale mechanisms that lead to the stresses and fracture patterns discussed in the preceding section. The key requirement for reactions to take place is that water has access to the reactive surface. In the examples given below, we will show how fracturing c ...
... We will now take a closer look at the pore-scale mechanisms that lead to the stresses and fracture patterns discussed in the preceding section. The key requirement for reactions to take place is that water has access to the reactive surface. In the examples given below, we will show how fracturing c ...
Electrochemical Reduction of Indium Tin Oxide (ITO)
... anions of the solution. The reduction of ITO is favoured at low pH, because protons are involved in the reduction process. NO3- ions are found to significantly inhibit the reduction of ITO, although the reason is still unclear. The potential at which ITO starts irreversible reduction (turns dark) va ...
... anions of the solution. The reduction of ITO is favoured at low pH, because protons are involved in the reduction process. NO3- ions are found to significantly inhibit the reduction of ITO, although the reason is still unclear. The potential at which ITO starts irreversible reduction (turns dark) va ...
Chapter 25
... • Most of the circuits analyzed will be assumed to be in steady state, with constant magnitude and direction • Because the potential difference between the terminals of a battery is constant, the battery produces direct current • The battery is known as a source of emf ...
... • Most of the circuits analyzed will be assumed to be in steady state, with constant magnitude and direction • Because the potential difference between the terminals of a battery is constant, the battery produces direct current • The battery is known as a source of emf ...
CHAPTER 1
... Refer to Figure 10.34a in the book. (a) If vin (t ) 0, we have only a dc source in the circuit. In steady state, the capacitor acts as an open circuit. Then we see that D2 is forward conducting and D1 is in reverse breakdown. Allowing 0.6 V for the forward diode voltage the output voltage is -5 V. ...
... Refer to Figure 10.34a in the book. (a) If vin (t ) 0, we have only a dc source in the circuit. In steady state, the capacitor acts as an open circuit. Then we see that D2 is forward conducting and D1 is in reverse breakdown. Allowing 0.6 V for the forward diode voltage the output voltage is -5 V. ...
Estimate the strength of given sodium carbonate solution
... standardised first by primary standard solution and then used as a secondary standard solution. Indicators: Indicators are the compounds which show the colour change at end point. ...
... standardised first by primary standard solution and then used as a secondary standard solution. Indicators: Indicators are the compounds which show the colour change at end point. ...
AP Electrostatics
... To prevent flash over, a series of foil sections are attached to the center portion of each disk and equally spaced and back to back with foil sections on the outer sides. To remove the charge, collection arms are arranged to collect the charge and transfer the charge to a storage capacitor. At 45 d ...
... To prevent flash over, a series of foil sections are attached to the center portion of each disk and equally spaced and back to back with foil sections on the outer sides. To remove the charge, collection arms are arranged to collect the charge and transfer the charge to a storage capacitor. At 45 d ...
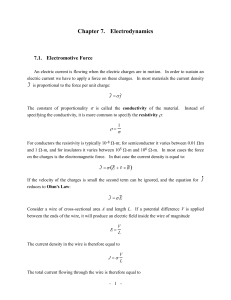
Chapter 7. Electrodynamics 7.1. Electromotive Force
... In any electric circuit a current will only exist if a driving force is available. The most common sources of the driving force are batteries and generators. When a circuit is hooked up to a power source a current will start to flow. In a single-loop circuit the current will be the same everywhere. ...
... In any electric circuit a current will only exist if a driving force is available. The most common sources of the driving force are batteries and generators. When a circuit is hooked up to a power source a current will start to flow. In a single-loop circuit the current will be the same everywhere. ...
Millikan`s Idea Robert Millikan was a scientist who studied electricity
... • “Stokes’ Theorem” – mass of droplets • How to find the electric field between two plates ...
... • “Stokes’ Theorem” – mass of droplets • How to find the electric field between two plates ...
AP Chemistry - luckyscience
... All compounds are electrically neutral. The sum of the positive and negative charges must add up to zero. ...
... All compounds are electrically neutral. The sum of the positive and negative charges must add up to zero. ...
How to Make Linear Mode Work
... Since the transfer characteristic crossover point is at a relatively high current, linear mode operation is almost always in the thermally unstable area below the crossover point. The problem is that the hotter areas on the die have higher current density, thus reinforcing hot spotting. Every MOSFET ...
... Since the transfer characteristic crossover point is at a relatively high current, linear mode operation is almost always in the thermally unstable area below the crossover point. The problem is that the hotter areas on the die have higher current density, thus reinforcing hot spotting. Every MOSFET ...
An Introduction to Chemistry
... enough to be seen with an optical microscope is considered to be microscopic. • MACROSCOPIC: Anything that is large enough to be seen with the naked eye is considered to be macroscopic. ...
... enough to be seen with an optical microscope is considered to be microscopic. • MACROSCOPIC: Anything that is large enough to be seen with the naked eye is considered to be macroscopic. ...
First Semester Final Review
... 46. What is the final concentration of barium ions, [Ba2+], in solution when 100. mL of 0.10 M BaCl2(aq) is mixed with 100. mL of 0.050 M H2SO4(aq)? a. 0.00 M b. 0.012 M c. 0.025 M d. 0.075 M e. 0.10 M 47. When 100 mL of 1.0 M Na3PO4 is mixed with 100 mL of 1.0 M AgNO3, a yellow precipitate forms wh ...
... 46. What is the final concentration of barium ions, [Ba2+], in solution when 100. mL of 0.10 M BaCl2(aq) is mixed with 100. mL of 0.050 M H2SO4(aq)? a. 0.00 M b. 0.012 M c. 0.025 M d. 0.075 M e. 0.10 M 47. When 100 mL of 1.0 M Na3PO4 is mixed with 100 mL of 1.0 M AgNO3, a yellow precipitate forms wh ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.