Handout #2 - MSU Billings
... Norm. "Well you see, Norm, it's like this... A herd of buffalo can only move as fast as the slowest buffalo. And when the herd is hunted, it's the slowest and weakest ones at the back that are killed first. This natural selection is good for the herd as a whole, because the general speed and health ...
... Norm. "Well you see, Norm, it's like this... A herd of buffalo can only move as fast as the slowest buffalo. And when the herd is hunted, it's the slowest and weakest ones at the back that are killed first. This natural selection is good for the herd as a whole, because the general speed and health ...
05- macromolecules - Kenston Local Schools
... Figure 5.4 (a) Linear and ring forms. Chemical equilibrium between the linear and ring structures greatly favors the formation of rings. To form the glucose ring, carbon 1 bonds to the oxygen attached to carbon 5. ...
... Figure 5.4 (a) Linear and ring forms. Chemical equilibrium between the linear and ring structures greatly favors the formation of rings. To form the glucose ring, carbon 1 bonds to the oxygen attached to carbon 5. ...
September 27 AP Biology - John D. O`Bryant School of Math & Science
... membrane that is impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 M sucrose solution. At 10-minute intervals, the bags were massed (weighed) and the percent change in mass of each ...
... membrane that is impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 M sucrose solution. At 10-minute intervals, the bags were massed (weighed) and the percent change in mass of each ...
Pre Ch13 HW
... Why gases have relatively low solubilities in water (§13.1) General characteristics of solutions formed by various combinations of gases, liquids, and solids (§13.1) How intermolecular forces stabilize the structures of proteins, the cell membrane, and DNA (§13.2) The enthalpy components of a soluti ...
... Why gases have relatively low solubilities in water (§13.1) General characteristics of solutions formed by various combinations of gases, liquids, and solids (§13.1) How intermolecular forces stabilize the structures of proteins, the cell membrane, and DNA (§13.2) The enthalpy components of a soluti ...
THE MOLE (pp. 159
... 3. The key is to convert the percent composition data to _______________. 4. Then compare the mole amounts to find the simplest whole number ratio. The ratio tells you how many atoms of each element are in the chemical formula. ***** Determine the chemical formula of a compound formed by mercury and ...
... 3. The key is to convert the percent composition data to _______________. 4. Then compare the mole amounts to find the simplest whole number ratio. The ratio tells you how many atoms of each element are in the chemical formula. ***** Determine the chemical formula of a compound formed by mercury and ...
STOICHIOMETRY:
... The word stoichiometry derives from two Greek words: stoicheion (meaning "element") and metron (meaning "measure"). Stoichiometry deals with calculations about the masses, volumes or concentrations of reactants and products involved in a chemical reaction. The reason we balance chemical reactions is ...
... The word stoichiometry derives from two Greek words: stoicheion (meaning "element") and metron (meaning "measure"). Stoichiometry deals with calculations about the masses, volumes or concentrations of reactants and products involved in a chemical reaction. The reason we balance chemical reactions is ...
Monitoring Reactions by TLC The fastest and most commonly used
... Monitoring Reactions by TLC The fastest and most commonly used method to follow the course of an organic reaction is by thin layer chromatography (TLC). If performed properly, one can use this simple technique to (1) determine the presence of starting material in the reaction (2) detect and monitor ...
... Monitoring Reactions by TLC The fastest and most commonly used method to follow the course of an organic reaction is by thin layer chromatography (TLC). If performed properly, one can use this simple technique to (1) determine the presence of starting material in the reaction (2) detect and monitor ...
Chapter 7 Chemical Quatities
... If you know the empirical formula and the molar mass, you can determine the molecular ...
... If you know the empirical formula and the molar mass, you can determine the molecular ...
Some basic concepts of chemistry
... DEFINITE COMPOSITION (JOSEPH PROUST) Statement: A given chemical compound always contains the same elements combined together in definite proportion by mass, i.e., it has a fixed composition and does not depend on the method of its preparation or the source from which it has been obtained. This law ...
... DEFINITE COMPOSITION (JOSEPH PROUST) Statement: A given chemical compound always contains the same elements combined together in definite proportion by mass, i.e., it has a fixed composition and does not depend on the method of its preparation or the source from which it has been obtained. This law ...
Loeblein chemistry clicker questions2013
... balloon is filled with Helium. How does the pressure of the air balloon compare to the pressure of the Helium balloon. The pressure in the air balloon is ...
... balloon is filled with Helium. How does the pressure of the air balloon compare to the pressure of the Helium balloon. The pressure in the air balloon is ...
LAB: (Day 1) Macromolecules/Enzymes
... When we eat, we consume macromolecules, vitamins, and minerals needed for our body to function normally. When macromolecules are consumed, it is necessary to break them down into smaller monomers to use them. Carbohydrates are broken down into simple sugars, such as glucose, that are used to create ...
... When we eat, we consume macromolecules, vitamins, and minerals needed for our body to function normally. When macromolecules are consumed, it is necessary to break them down into smaller monomers to use them. Carbohydrates are broken down into simple sugars, such as glucose, that are used to create ...
Chemistry Standards and Frameworks
... of space centered around a tiny nucleus, and so it is this region that defines the volume of the atom. If the nucleus (proton) of a hydrogen atom were as large as the width of a human thumb, the electron would be on the average about one kilometer away in a great expanse of empty space. The electro ...
... of space centered around a tiny nucleus, and so it is this region that defines the volume of the atom. If the nucleus (proton) of a hydrogen atom were as large as the width of a human thumb, the electron would be on the average about one kilometer away in a great expanse of empty space. The electro ...
pages 46-50
... Proteins are the most varied of the carbon-based molecules in organisms. In movement, eyesight, or digestion, proteins are at work. A protein is a polymer made of monomers called amino acids. Amino acids are molecules that contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur. Organisms u ...
... Proteins are the most varied of the carbon-based molecules in organisms. In movement, eyesight, or digestion, proteins are at work. A protein is a polymer made of monomers called amino acids. Amino acids are molecules that contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur. Organisms u ...
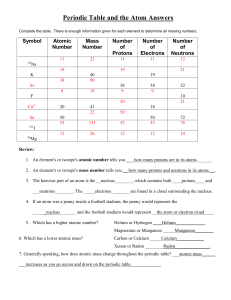
Periodic Table and the Atom Answers
... b) Arrange the pH in increasing order of hydrogen ion comcentration. 2) Define the term "pH"; what does" pH" stand for? Answer: The term "pH" is defined as the negative logarithm of H+ ion concentration of a given solution; the concentration being expressed as moles per litre. Mathematically pH = - ...
... b) Arrange the pH in increasing order of hydrogen ion comcentration. 2) Define the term "pH"; what does" pH" stand for? Answer: The term "pH" is defined as the negative logarithm of H+ ion concentration of a given solution; the concentration being expressed as moles per litre. Mathematically pH = - ...
Macromolecules Biological Molecules Macromolecules
... Proteins: Environmental conditions affect protein structure Some Conditions can not break covalent bonds, but can upset the weaker noncovalent interactions that determine secondary and tertiary structure, may affect a protein's shape and thus its function Denaturation Couses: Increases in tempe ...
... Proteins: Environmental conditions affect protein structure Some Conditions can not break covalent bonds, but can upset the weaker noncovalent interactions that determine secondary and tertiary structure, may affect a protein's shape and thus its function Denaturation Couses: Increases in tempe ...
Carefully detach the last page. It is the Data Sheet.
... 13 When ethanol, CH3CH2OH, is burned in excess oxygen, carbon dioxide and water are the only products. What is the coefficient of O2 when the chemical equation 17 When the hydrides of the group 16 elements are representing the combustion reaction is balanced using arranged in order of increasing boi ...
... 13 When ethanol, CH3CH2OH, is burned in excess oxygen, carbon dioxide and water are the only products. What is the coefficient of O2 when the chemical equation 17 When the hydrides of the group 16 elements are representing the combustion reaction is balanced using arranged in order of increasing boi ...
Model 2 – Amylase Rate of Reaction
... Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help remove stains from our shirts, turn milk into cheese, and are responsible for turning our dinner into use- able fuel for our bodies. Enzymes however do not work well universally. Som ...
... Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help remove stains from our shirts, turn milk into cheese, and are responsible for turning our dinner into use- able fuel for our bodies. Enzymes however do not work well universally. Som ...
practice problems of chap4_5 - Chemistry
... reaction involves electron transfer, which leads to change of oxidation number of the elements. You need first to figure out the oxidation number of each species. The reaction that does not have change in oxidatio ... pptx | 3,9 MB | 59 pages doc ... need to get the molar mass of the compound first. ...
... reaction involves electron transfer, which leads to change of oxidation number of the elements. You need first to figure out the oxidation number of each species. The reaction that does not have change in oxidatio ... pptx | 3,9 MB | 59 pages doc ... need to get the molar mass of the compound first. ...
Semester Exam Review
... molar, if the initial reaction concentrations are [X]o = 0.80 molar, [Y]o = 0.60 molar and [Z]0 = 0 molar? (d) Select from the mechanisms below the one most consistent with the observed data, and explain your choice. In these mechanisms M and N are reaction intermediates. (1) X + Y M (slow) X+MZ ...
... molar, if the initial reaction concentrations are [X]o = 0.80 molar, [Y]o = 0.60 molar and [Z]0 = 0 molar? (d) Select from the mechanisms below the one most consistent with the observed data, and explain your choice. In these mechanisms M and N are reaction intermediates. (1) X + Y M (slow) X+MZ ...
protein - Portal UniMAP
... Organic solvents – water-soluble organic solvents eg. Ethanol interfere with hydrophobic interaction because they interact with nonpolar R groups and form H bond with water and polar protein group. ...
... Organic solvents – water-soluble organic solvents eg. Ethanol interfere with hydrophobic interaction because they interact with nonpolar R groups and form H bond with water and polar protein group. ...
File
... 6. Matter is anything that has a mass and takes up space. An element is the simplest form of matter, which cannot be broken down any further. Elements are listed on Table S and the periodic table. Their symbols start with an uppercase letter. a. Which of the following is not matter? ________________ ...
... 6. Matter is anything that has a mass and takes up space. An element is the simplest form of matter, which cannot be broken down any further. Elements are listed on Table S and the periodic table. Their symbols start with an uppercase letter. a. Which of the following is not matter? ________________ ...
Unit 2 – Quantities Review
... If values are not close to a real number multiply all values by the same coefficient to end up with whole numbers. Ex. If the ratio was 1:2:1.33, multiply all numbers by 3 to get 1:6:4 Practice Problems 22. The percentage compositions of two antibiotics are given below. Find the empirical formula ...
... If values are not close to a real number multiply all values by the same coefficient to end up with whole numbers. Ex. If the ratio was 1:2:1.33, multiply all numbers by 3 to get 1:6:4 Practice Problems 22. The percentage compositions of two antibiotics are given below. Find the empirical formula ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.