COVENANT UNIVERSITY College of Science and Technology
... A selection of experiments designed to provide illustrations of the important parts of the lectures in CHM226 Course. The experiments will afford the students the opportunity to develop their quantitative and analytical skills. Topics include chemical equilibria, kinetics of iodination of acetone, d ...
... A selection of experiments designed to provide illustrations of the important parts of the lectures in CHM226 Course. The experiments will afford the students the opportunity to develop their quantitative and analytical skills. Topics include chemical equilibria, kinetics of iodination of acetone, d ...
Chapter 17 lecture notes on Chemical Equilibria
... Extracting kinetics from the plot: This plot provides us with enormous information about both the kinetics and the thermodynamics of the reaction. From a kinetic perspectives we know that the reaction proceeds at a rate determined by ...
... Extracting kinetics from the plot: This plot provides us with enormous information about both the kinetics and the thermodynamics of the reaction. From a kinetic perspectives we know that the reaction proceeds at a rate determined by ...
AP Chemistry
... I have taught AP Chemistry for 10 years and am very excited about next year. AP Chemistry is designed to prepare you to be successful in college chemistry as well as to pass the AP Chemistry test. Attached is the summer work placket to prepare you. Expect a test on this material the second day of sc ...
... I have taught AP Chemistry for 10 years and am very excited about next year. AP Chemistry is designed to prepare you to be successful in college chemistry as well as to pass the AP Chemistry test. Attached is the summer work placket to prepare you. Expect a test on this material the second day of sc ...
C2 Revision Quick Questions FT
... other because there are no covalent bonds between the layers and so graphite is soft and ...
... other because there are no covalent bonds between the layers and so graphite is soft and ...
C2 Revision Quick Questions FT
... other because there are no covalent bonds between the layers and so graphite is soft and ...
... other because there are no covalent bonds between the layers and so graphite is soft and ...
KINETICS questions
... (a) Complete the potential-energy diagram for reaction II on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 20C. Explain the basis for each prediction. (i) Rate of reaction (ii) Heat of reaction (c) For reaction II, the form of ...
... (a) Complete the potential-energy diagram for reaction II on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 20C. Explain the basis for each prediction. (i) Rate of reaction (ii) Heat of reaction (c) For reaction II, the form of ...
Activity C14: Rate of a Chemical Reaction 1
... to use a Colorimeter to measure the formation of the solid sulfur generated. The solid sulfur will block the light in the Colorimeter and the amount of blockage is directly proportional to the amount of sulfur in suspension. The rate of this chemical reaction is given by the equation: Rate = k [thio ...
... to use a Colorimeter to measure the formation of the solid sulfur generated. The solid sulfur will block the light in the Colorimeter and the amount of blockage is directly proportional to the amount of sulfur in suspension. The rate of this chemical reaction is given by the equation: Rate = k [thio ...
AP Chemistry
... A(g) + B(g) C(g) + D(g) For the gas-phase reaction represented above, the following experimental data were obtained. Exp. Initial [A] Initial [B] ...
... A(g) + B(g) C(g) + D(g) For the gas-phase reaction represented above, the following experimental data were obtained. Exp. Initial [A] Initial [B] ...
ACP Chemistry Semester 1 Final Exam - Doc-U-Ment
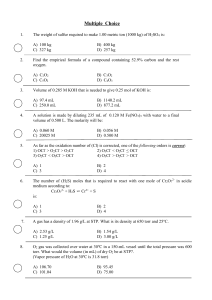
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
Are You suprised ?
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
Lecture 14
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
3. What is the empirical formula of a compound that is
... 6. Suppose that there is 1 L of O2 in your blood and excess C6H12O6. How many grams of water will form if all of the oxygen is used in the process of respiration? ...
... 6. Suppose that there is 1 L of O2 in your blood and excess C6H12O6. How many grams of water will form if all of the oxygen is used in the process of respiration? ...
Ch 9 Pkt - mvhs
... 13. A sample of Calcium chloride (CaCl2) has a mass of 23.8 g. How many moles of Calcium chloride is this? 14. How many grams are there in 0.36 moles of Cobalt (III) acetate (Co(C2H3O2)3)? How many grams of cobalt are in this sample? How many atoms of cobalt? 15. How many mg of chlorine are there in ...
... 13. A sample of Calcium chloride (CaCl2) has a mass of 23.8 g. How many moles of Calcium chloride is this? 14. How many grams are there in 0.36 moles of Cobalt (III) acetate (Co(C2H3O2)3)? How many grams of cobalt are in this sample? How many atoms of cobalt? 15. How many mg of chlorine are there in ...
Describing Chemical Reactions
... The principle called conservation of mass was first demonstrated in the late 1700s. The principle of conservation of mass states that in a chemical reaction, the total mass of the reactants must equal the total mass of the products. In an open system, matter can enter from or escape to the surroundi ...
... The principle called conservation of mass was first demonstrated in the late 1700s. The principle of conservation of mass states that in a chemical reaction, the total mass of the reactants must equal the total mass of the products. In an open system, matter can enter from or escape to the surroundi ...
NZIC 2012 - Rangiora High School
... Since HA is a strong acid, it reacts completely with water giving a 0.1 mol L–1 solution, so its conductivity will be high. HA + H2O A– + H3O+ Since HB is a weak acid, reaction with water is incomplete giving a solution that is ~ 10–3 mol L–1 in ions so its conductivity will be lower than that of ...
... Since HA is a strong acid, it reacts completely with water giving a 0.1 mol L–1 solution, so its conductivity will be high. HA + H2O A– + H3O+ Since HB is a weak acid, reaction with water is incomplete giving a solution that is ~ 10–3 mol L–1 in ions so its conductivity will be lower than that of ...
Reaction Rates/Chemical Kinetics
... For the equation, N2 + H2 NH3 It doesn’t matter whether we start with N2 and H2 or whether we start with NH3: we will have the same proportions of all three substances at ...
... For the equation, N2 + H2 NH3 It doesn’t matter whether we start with N2 and H2 or whether we start with NH3: we will have the same proportions of all three substances at ...
Example - Schoolwires.net
... must know the mass of each isotope and the atom percent of the isotopes ...
... must know the mass of each isotope and the atom percent of the isotopes ...
6.02 × 1023 molecules = 1 mole
... convert mass percent of each element to a molar ratio of each element. Step 1 Assume your unknown sample has a mass of 100 grams. ...
... convert mass percent of each element to a molar ratio of each element. Step 1 Assume your unknown sample has a mass of 100 grams. ...