Practice Test_final_161_F2015
... 1. A 19.0-g sample of lithium is completely burned in air to form lithium oxide. The mass of lithium oxide must be A) less than 19.0 g. B) greater than 19.0 g. C) equal to 19.0 g. D) all of the above. E) none of the above. ...
... 1. A 19.0-g sample of lithium is completely burned in air to form lithium oxide. The mass of lithium oxide must be A) less than 19.0 g. B) greater than 19.0 g. C) equal to 19.0 g. D) all of the above. E) none of the above. ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... and at a constant temperature, the ratio of reactant and product concentrations has a constant value, k (the equilibrium constant). • Note that although the concentrations may vary, as long as a given reaction is at equilibrium and the temperature does not change, according to the law of mass action ...
... and at a constant temperature, the ratio of reactant and product concentrations has a constant value, k (the equilibrium constant). • Note that although the concentrations may vary, as long as a given reaction is at equilibrium and the temperature does not change, according to the law of mass action ...
Predicting Moles of Reactants/Products
... 2. How many moles of MgO form when 3 moles of O2 react? Answer: _____ 3. How many moles of MgO form when 8 moles of Mg react? Answer: ______ 4. How many moles of O2 are required to produce 20 moles of MgO? ...
... 2. How many moles of MgO form when 3 moles of O2 react? Answer: _____ 3. How many moles of MgO form when 8 moles of Mg react? Answer: ______ 4. How many moles of O2 are required to produce 20 moles of MgO? ...
Contents - MCAT Prep Course
... An increase in the attractive intermolecular forces necessitates a greater amount of energy for a liquid molecule to escape to the vapour phase. Therefore, vapour pressure decreases ...
... An increase in the attractive intermolecular forces necessitates a greater amount of energy for a liquid molecule to escape to the vapour phase. Therefore, vapour pressure decreases ...
chemistry - Textbooks Online
... Alchemy was a mixture of scientific investigation and mystical quest, with strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), an ...
... Alchemy was a mixture of scientific investigation and mystical quest, with strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), an ...
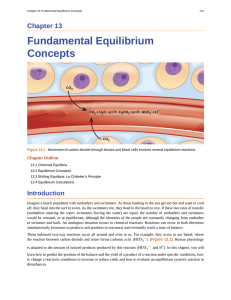
Fundamental Equilibrium Concepts
... exhausted and will reverse only under certain conditions. Such reactions are often depicted with a one-way arrow from reactants to products. Many other reactions, such as the formation of NO2 from N2O4, are reversible under more easily obtainable conditions and, therefore, are named as such. In a re ...
... exhausted and will reverse only under certain conditions. Such reactions are often depicted with a one-way arrow from reactants to products. Many other reactions, such as the formation of NO2 from N2O4, are reversible under more easily obtainable conditions and, therefore, are named as such. In a re ...
msc_pre_chemistry_pap1_bl2
... energies, (ii) higher LF or MO splittings. The µ at room temperature is generally lower than µs and cannot be used to determine the unpaired electrons due to (iii) high spin-orbit coupling constants which align L and S vectors in opposite directions destroying the paramagnetism. Further, (iv) the Cu ...
... energies, (ii) higher LF or MO splittings. The µ at room temperature is generally lower than µs and cannot be used to determine the unpaired electrons due to (iii) high spin-orbit coupling constants which align L and S vectors in opposite directions destroying the paramagnetism. Further, (iv) the Cu ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...