template
... According to the table you just made, How many molecules of ammonia can be produced? Which reactant is in excess? Why? How many molecules of excess reactant are there? Describe what you must look for in a particular reactant mixture to decide which reactant will be in excess (have some left over aft ...
... According to the table you just made, How many molecules of ammonia can be produced? Which reactant is in excess? Why? How many molecules of excess reactant are there? Describe what you must look for in a particular reactant mixture to decide which reactant will be in excess (have some left over aft ...
Cheat Sheet for Chemical Equilibrium
... • Given: Initial Concentrations and asked whether a precipitate will form: Calculate Q (no ICE chart needed) and compare with Ksp: o Q>Ksp, precipitate will form o Q=Ksp, at equilibrium o Q
... • Given: Initial Concentrations and asked whether a precipitate will form: Calculate Q (no ICE chart needed) and compare with Ksp: o Q>Ksp, precipitate will form o Q=Ksp, at equilibrium o Q
MCQ plus answers
... An enzyme is a protein that is a highly efficient catalyst for one or more chemical reactions in a living system. ...
... An enzyme is a protein that is a highly efficient catalyst for one or more chemical reactions in a living system. ...
Student Exploration Sheet: Growing Plants
... Username – btesta Password - science Vocabulary: coefficient, combination, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript ...
... Username – btesta Password - science Vocabulary: coefficient, combination, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript ...
7.2 Writing Chemical Equations
... substances change in his atomic theory. In a chemical reaction the ways in which the atoms are joined together are changed. As reactants are changed into products, bonds that hold atoms together are broken and new bonds are ...
... substances change in his atomic theory. In a chemical reaction the ways in which the atoms are joined together are changed. As reactants are changed into products, bonds that hold atoms together are broken and new bonds are ...
2 - CronScience
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
... Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as an ionic equation by splitting the compounds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “ ...
Chemistry Final Review 2017 1. List a set of elements
... 8. What is the chemical formula for iron(III) oxide? 9. What is the chemical formula for copper(II) hydroxide? 10. What is the formula for the compound that forms when magnesium bonds with phosphorus? 11. What is the formula for calcium phosphate? 12. List all types of chemical reactions. 13. Give a ...
... 8. What is the chemical formula for iron(III) oxide? 9. What is the chemical formula for copper(II) hydroxide? 10. What is the formula for the compound that forms when magnesium bonds with phosphorus? 11. What is the formula for calcium phosphate? 12. List all types of chemical reactions. 13. Give a ...
Thermochemistry: The Heat of Neutralization
... Most physical and chemical changes are either endothermic or exothermic. Endothermic reactions absorb energy or heat from the surroundings and result in a lower temperature. These reactions will feel cool or cold to the touch because they are absorbing heat energy from your hand. Exothermic reaction ...
... Most physical and chemical changes are either endothermic or exothermic. Endothermic reactions absorb energy or heat from the surroundings and result in a lower temperature. These reactions will feel cool or cold to the touch because they are absorbing heat energy from your hand. Exothermic reaction ...
PDF(343KB)
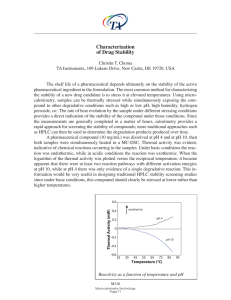
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
Topic 6 - uaschemistry
... The total mass of the reaction mixture will only vary if a gas is evolved. The gas should have a high molar mass (ie. not hydrogen) ...
... The total mass of the reaction mixture will only vary if a gas is evolved. The gas should have a high molar mass (ie. not hydrogen) ...
Week - Mat-Su School District
... Room 123 Description: This is an advanced Science course designed to prepare the student for either college Chemistry or AP Chemistry. The course covers the equivalent of one full year of general Chemistry, comparable to a first year course at a college or university. The course is a rigorous math-b ...
... Room 123 Description: This is an advanced Science course designed to prepare the student for either college Chemistry or AP Chemistry. The course covers the equivalent of one full year of general Chemistry, comparable to a first year course at a college or university. The course is a rigorous math-b ...