PAP Chemistry - Fall Final Review
... 6. What did Rutherford discover from the Gold Foil Experiment – p.72 The nucleus and that the atom was mostly empty space 7. When is a bright-line spectrum produced by an atom? IE – How does an atom give off color (especially when burned)? The resting state or the ground state is when the electron i ...
... 6. What did Rutherford discover from the Gold Foil Experiment – p.72 The nucleus and that the atom was mostly empty space 7. When is a bright-line spectrum produced by an atom? IE – How does an atom give off color (especially when burned)? The resting state or the ground state is when the electron i ...
activity series
... occurs between ions in aqueous solution. A reaction will occur when a pair of ions come together to produce at least one of the following: 1. a precipitate 2. a gas 3. water or some other non-ionized substance. ...
... occurs between ions in aqueous solution. A reaction will occur when a pair of ions come together to produce at least one of the following: 1. a precipitate 2. a gas 3. water or some other non-ionized substance. ...
Thermochemistry 2 Matching Match each item with the correct
... Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
... Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
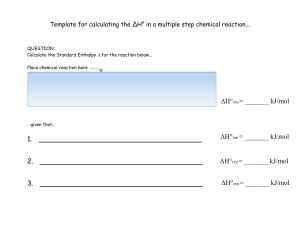
Template for calculating the ΔH° in a multiple step chemical reaction
... b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction The chemical reaction: Zn + H2SO4 → ZnSO4 + H2 is a a. synthesis reaction b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction ...
... b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction The chemical reaction: Zn + H2SO4 → ZnSO4 + H2 is a a. synthesis reaction b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction ...
STUDY GUIDE
... Chemicals should not be stored in alphabetical order because some chemicals that will react if mixed could end up next to each other. For example, acids should not be stored near cyanides, sulfides and other chemicals that produce toxic gases when combined. Acids should also not be stored near bases ...
... Chemicals should not be stored in alphabetical order because some chemicals that will react if mixed could end up next to each other. For example, acids should not be stored near cyanides, sulfides and other chemicals that produce toxic gases when combined. Acids should also not be stored near bases ...
e c n i
... b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
... b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
Wk-11-14
... Atomic Mass Unit. Pay attention—this is where Avogadro's number comes from. •Earlier we said "Let one atom of H have 1 atomic mass unit" •Now, we have a problem, because H has 3 isotopes: •So.....we cannot use "hydrogen" as it usually exists (mixed isotopes) for our mass standard. •We must purify i ...
... Atomic Mass Unit. Pay attention—this is where Avogadro's number comes from. •Earlier we said "Let one atom of H have 1 atomic mass unit" •Now, we have a problem, because H has 3 isotopes: •So.....we cannot use "hydrogen" as it usually exists (mixed isotopes) for our mass standard. •We must purify i ...
RTF
... 3. For the equilibrium system at a certain temperature, described by the equation PCl3(g) + Cl2(g) Keq = 60 ...
... 3. For the equilibrium system at a certain temperature, described by the equation PCl3(g) + Cl2(g) Keq = 60 ...
Physical Science
... b. When no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
... b. When no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
Journal of Physical and Chemical Reference Data
... molar standard enthalpies of formation of each product scaled by the coefficients in the balanced reaction for each product and from this sum over the products subtract a similar sum over the reactants, where the molar standard enthalpy of formation of each reactant is also scaled by the coefficient ...
... molar standard enthalpies of formation of each product scaled by the coefficients in the balanced reaction for each product and from this sum over the products subtract a similar sum over the reactants, where the molar standard enthalpy of formation of each reactant is also scaled by the coefficient ...
Spring 2014 Chemistry Review
... Bronsted-Lowry Theory Lewis Theory Name the following: 113) HC2H3O2:________________ 114) HF: __________________ 115) H2SO3: __________________ ...
... Bronsted-Lowry Theory Lewis Theory Name the following: 113) HC2H3O2:________________ 114) HF: __________________ 115) H2SO3: __________________ ...
Basic Background Review: Acid-Base , Redox, and Stable Isotopes
... •ΔH largely function of strengths of chemical bonds in products vs, reactant (more strong bonds in product than reactants (negative ΔH) contributes to spontaneous reaction) •ΔS measure of relative degree of disorder in product and reactant—greater disorder in product than reactant (positive ΔS) ...
... •ΔH largely function of strengths of chemical bonds in products vs, reactant (more strong bonds in product than reactants (negative ΔH) contributes to spontaneous reaction) •ΔS measure of relative degree of disorder in product and reactant—greater disorder in product than reactant (positive ΔS) ...
50 Forgotten Facts
... b) Adding a catalyst will _________________________ the activation energy by ___________________ steps from the reaction pathway (mechanism). c) Adding an inhibitor will __________________________ the activation energy by ___________________ steps to the reaction pathway. ...
... b) Adding a catalyst will _________________________ the activation energy by ___________________ steps from the reaction pathway (mechanism). c) Adding an inhibitor will __________________________ the activation energy by ___________________ steps to the reaction pathway. ...
fo-Balancing Chemical Notes
... element is an element that only occurs in one compound in the reactants and in one compound in the products. 3. Change the coefficients of the compounds containing the selected element so that the same number of atoms of this element occur on both the reactant and product sides of the equation. 4. G ...
... element is an element that only occurs in one compound in the reactants and in one compound in the products. 3. Change the coefficients of the compounds containing the selected element so that the same number of atoms of this element occur on both the reactant and product sides of the equation. 4. G ...
+ 2 O 2 - SandersScienceStuff
... 2 NaOH + 1 CaBr2 1 Ca(OH)2 + 2 NaBr What is the molar ratio between sodium hydroxide and calcium bromide in this equation? ...
... 2 NaOH + 1 CaBr2 1 Ca(OH)2 + 2 NaBr What is the molar ratio between sodium hydroxide and calcium bromide in this equation? ...