Print this article - Bangladesh Journals Online
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
Chapter 3 - Educator
... chapter, there are chemical changes occurring in you. The changes that occur in your eyes and brain, for example, are what allows you to see these words and think about them. Although such chemical changes are not as obvious as the reaction shown in the chapter-opening photograph, they are neverthel ...
... chapter, there are chemical changes occurring in you. The changes that occur in your eyes and brain, for example, are what allows you to see these words and think about them. Although such chemical changes are not as obvious as the reaction shown in the chapter-opening photograph, they are neverthel ...
CH4 Student Revision Guides pdf | GCE AS/A
... This means that benzene is more stable than expected, by approximately150 kJ mol . This energy difference arises from the delocalisation of the -electrons and is called the delocalisation energy or stabilisation energy. An older term was the resonance energy. ...
... This means that benzene is more stable than expected, by approximately150 kJ mol . This energy difference arises from the delocalisation of the -electrons and is called the delocalisation energy or stabilisation energy. An older term was the resonance energy. ...
1 Chemistry HP Unit 5 – Stoichiometry Learning Targets (Your exam
... 5-2. Determine the number of atoms of each element in a given compound from the chemical formula. 5-3. Define molar mass. 5-4. Determine the molar mass for a given element/compound with the appropriate units. What is a “mole?” The “mole” is an SI (System International) unit of measurement based on c ...
... 5-2. Determine the number of atoms of each element in a given compound from the chemical formula. 5-3. Define molar mass. 5-4. Determine the molar mass for a given element/compound with the appropriate units. What is a “mole?” The “mole” is an SI (System International) unit of measurement based on c ...
PDF - mockies – Mockiesgateacademy
... Where has chemistry come from ? Throughout the history of the human race, people have struggled to make sense of the world around them. Through the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on ...
... Where has chemistry come from ? Throughout the history of the human race, people have struggled to make sense of the world around them. Through the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on ...
Document
... series, the atomic radii actually ____________again. At the beginning of the series, the increase in __________ _______________ with atomic number pulls in the electron cloud, resulting in a reduction of atomic size. Since electrons are added to an inner d subshell across the series, this adds to th ...
... series, the atomic radii actually ____________again. At the beginning of the series, the increase in __________ _______________ with atomic number pulls in the electron cloud, resulting in a reduction of atomic size. Since electrons are added to an inner d subshell across the series, this adds to th ...
Review Unit: Chemistry Review
... Science involves describing, predicting, and explaining nature and its changes in the simplest way possible. Scientists refine the descriptions of the natural world so that these descriptions are as precise and complete as possible. In science, reliable and accurate descriptions of phenomena become ...
... Science involves describing, predicting, and explaining nature and its changes in the simplest way possible. Scientists refine the descriptions of the natural world so that these descriptions are as precise and complete as possible. In science, reliable and accurate descriptions of phenomena become ...
Mass Relationships in Chemical Reactions
... percentage can be converted directly to grams. In this sample, there will be 40.92 g of C, 4.58 g of H, and 54.50 g of O. Because the subscripts in the formula represent a mole ratio, we need to convert the grams of each element to moles. The conversion factor needed is the molar mass of each elemen ...
... percentage can be converted directly to grams. In this sample, there will be 40.92 g of C, 4.58 g of H, and 54.50 g of O. Because the subscripts in the formula represent a mole ratio, we need to convert the grams of each element to moles. The conversion factor needed is the molar mass of each elemen ...
1. Bromine exists naturally as a mixture of bromine
... atomic mass 114.8 g. The nucleus of an atom of indium-112 contains A) 49 protons, 63 neutrons, 49 electrons. B) 49 protons, 49 neutrons. C) 49 protons, 49 alpha particles. D) 49 protons, 63 neutrons. E) 49 protons, 112 neutrons. Gallium consists of two isotopes of masses 68.95 amu and 70.95 amu with ...
... atomic mass 114.8 g. The nucleus of an atom of indium-112 contains A) 49 protons, 63 neutrons, 49 electrons. B) 49 protons, 49 neutrons. C) 49 protons, 49 alpha particles. D) 49 protons, 63 neutrons. E) 49 protons, 112 neutrons. Gallium consists of two isotopes of masses 68.95 amu and 70.95 amu with ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... observable and measureable properties can be used to classify and describe matter and energy. Eligible Content CHEM.A.1.1.5 – Apply systematic set of rules (IUPAC) for naming compounds and writing chemical formulas (e.g., binary covalent binary ionic, ionic compounds containing polyatomic ions). S ...
... observable and measureable properties can be used to classify and describe matter and energy. Eligible Content CHEM.A.1.1.5 – Apply systematic set of rules (IUPAC) for naming compounds and writing chemical formulas (e.g., binary covalent binary ionic, ionic compounds containing polyatomic ions). S ...
C7 Revision Notes 2015
... This was an oversimplification! Some reactions are Note the different arrow used quite easily reversed.... REVERSIBLE typeforofreversible REACTIONS reactions N2(g) +reactions 3H2(g) and 2NH Reversible equilibria 3(g) ...
... This was an oversimplification! Some reactions are Note the different arrow used quite easily reversed.... REVERSIBLE typeforofreversible REACTIONS reactions N2(g) +reactions 3H2(g) and 2NH Reversible equilibria 3(g) ...
Observation of Nonlinear Optical Interactions of Ultralow Levels of Light
... created a number of advancements in generating and controlling classical and quantum states of light [4 –6]. In order to enable optical interactions with few photons (as desired in some quantum information processing studies [7]), much effort has been invested into increasing the interaction between ...
... created a number of advancements in generating and controlling classical and quantum states of light [4 –6]. In order to enable optical interactions with few photons (as desired in some quantum information processing studies [7]), much effort has been invested into increasing the interaction between ...
Program Review - Austin Community College
... The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught in the lectures and labs (for example, Nuclear Magnetic Resonance and Infrared simulation programs, ...
... The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught in the lectures and labs (for example, Nuclear Magnetic Resonance and Infrared simulation programs, ...
College Grossmont 115
... or numbers obtained by definition. For example, we can count the fingers on our hand and get an exact number (most people have 5). There is no uncertainty in this result, but we cannot count large groups of objects without some degree of uncertainty. For example, the number of stars in our galaxy is ...
... or numbers obtained by definition. For example, we can count the fingers on our hand and get an exact number (most people have 5). There is no uncertainty in this result, but we cannot count large groups of objects without some degree of uncertainty. For example, the number of stars in our galaxy is ...
Official Drugstore. Can You Take Cialis With Lisinopril
... How many grams of carbon dioxide are produced by the complete combustion of 100. g of pentane (C5H12)? ...
... How many grams of carbon dioxide are produced by the complete combustion of 100. g of pentane (C5H12)? ...
Chapter 4 - Fort Bend ISD
... Dalton’s Atomic Theory Based on Experiments • John Dalton published his atomic theory in 1803. • His theory stated •all substances are made of atoms. •Atoms are small particles that cannot be created, divided, or destroyed. •Atoms of the same element are exactly alike, and atoms of different element ...
... Dalton’s Atomic Theory Based on Experiments • John Dalton published his atomic theory in 1803. • His theory stated •all substances are made of atoms. •Atoms are small particles that cannot be created, divided, or destroyed. •Atoms of the same element are exactly alike, and atoms of different element ...
21:3 Classifying Chemical Reactions
... in molecules by very strong triple bonds. In order to be used by living things, these triple bonds need to be broken. Lightning can supply energy that frees nitrogen atoms to form new compounds. ...
... in molecules by very strong triple bonds. In order to be used by living things, these triple bonds need to be broken. Lightning can supply energy that frees nitrogen atoms to form new compounds. ...
Differentiated Chemistry Worksheet and Laboratory
... Short Answer. Answer the following questions. We spend quite a bit of time staring at the red and green lights in a traffic signals. While that image is in your mind, for questions 92 – 95, decide which would have the following for a red light with a 4.41 x 1014 Hz frequency or a green light with a ...
... Short Answer. Answer the following questions. We spend quite a bit of time staring at the red and green lights in a traffic signals. While that image is in your mind, for questions 92 – 95, decide which would have the following for a red light with a 4.41 x 1014 Hz frequency or a green light with a ...
1 of 52
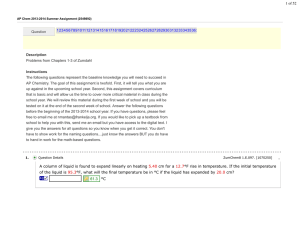
... AP Chemistry. The goal of this assignment is twofold. First, it will tell you what you are up against in the upcoming school year. Second, this assignment covers curriculum that is basic and will allow us the time to cover more critical material in class during the school year. We will review this m ...
... AP Chemistry. The goal of this assignment is twofold. First, it will tell you what you are up against in the upcoming school year. Second, this assignment covers curriculum that is basic and will allow us the time to cover more critical material in class during the school year. We will review this m ...
AVOGADRO EXAMS 1991 - 2002 PRACTICE BOOKLET
... 14. ”A valence electron in a pure metal is not held by any specific atom, but all valence electrons are used to hold together the atoms of the metal.” This statement is best classified as (a) a specific experimental fact (b) an opinion not based upon evidence (c) a correct definition of a chemical t ...
... 14. ”A valence electron in a pure metal is not held by any specific atom, but all valence electrons are used to hold together the atoms of the metal.” This statement is best classified as (a) a specific experimental fact (b) an opinion not based upon evidence (c) a correct definition of a chemical t ...
Hybridization of atomic orbitals
... Atomic orbitals are (energy) states or wave forms of electrons in the atom. If we insist on the particle nature of electrons, then the probability of finding an electron in an atomic orbital is proportional to the square of the wavefunction. The values of the wavefunction can be either positive or n ...
... Atomic orbitals are (energy) states or wave forms of electrons in the atom. If we insist on the particle nature of electrons, then the probability of finding an electron in an atomic orbital is proportional to the square of the wavefunction. The values of the wavefunction can be either positive or n ...
1.6 Energy changes in chemical reactions
... Chemists deal with matter on a macroscopic scale in the laboratory, but explain its behaviour in terms of atoms and molecules. This requires a wide range of distances (see Figure 1.4). You will need to become familiar with the multiplication prefixes in Table 1.3 used to describe lengths on atomic a ...
... Chemists deal with matter on a macroscopic scale in the laboratory, but explain its behaviour in terms of atoms and molecules. This requires a wide range of distances (see Figure 1.4). You will need to become familiar with the multiplication prefixes in Table 1.3 used to describe lengths on atomic a ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.