CHEMISTRY 2202
... electronegativity between the H and Cl. The shared pair of electrons is partially pulled toward the more electronegative Cl resulting in a charge separation between the H and Cl. The overall effect is a polar bond toward the Cl. ...
... electronegativity between the H and Cl. The shared pair of electrons is partially pulled toward the more electronegative Cl resulting in a charge separation between the H and Cl. The overall effect is a polar bond toward the Cl. ...
chapter - Max-Planck-Institut für Astronomie
... of deuterium with respect to hydrogen in molecules. Although deuterium atoms are only ∼ 1.6 × 10−5 (Tab. 1) times as abundant as the hydrogen atoms in the Universe, its relative abundance in molecules, larger than the elemental D/H abundance in very specific situations, provides a remarkable and alm ...
... of deuterium with respect to hydrogen in molecules. Although deuterium atoms are only ∼ 1.6 × 10−5 (Tab. 1) times as abundant as the hydrogen atoms in the Universe, its relative abundance in molecules, larger than the elemental D/H abundance in very specific situations, provides a remarkable and alm ...
Enthalpy change
... Standard Enthalpy Changes • enthalpy values vary with the conditions - so standard conditions are needed • a substance will then be in its standard state ... Pressure:- 100 kPa (1 atm) ...
... Standard Enthalpy Changes • enthalpy values vary with the conditions - so standard conditions are needed • a substance will then be in its standard state ... Pressure:- 100 kPa (1 atm) ...
Worked solutions to the problems
... materials that your students use and produce. Students should of course also make themselves aware of any hazards associated with the chemicals that they will be using in any exercise and we encourage you to bring these to their attention. For our junior colleagues who will spend many hours over the ...
... materials that your students use and produce. Students should of course also make themselves aware of any hazards associated with the chemicals that they will be using in any exercise and we encourage you to bring these to their attention. For our junior colleagues who will spend many hours over the ...
Document
... Molecular (Covalent) Compounds Covalent compounds contain nonmetals that “share” electrons to form molecules. (molecular compounds) ...
... Molecular (Covalent) Compounds Covalent compounds contain nonmetals that “share” electrons to form molecules. (molecular compounds) ...
Stoichiometery
... Real Chemistry Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
... Real Chemistry Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
Chemistry 133 Problem Set Introduction
... 1.81 Antifreeze contains the compound ethylene glycol. This compound not only lowers the freezing point of water but also increases the boiling point of water. The density of ethylene glycol is 9.35 lb/gal, and the density of water is 62.5 lb/ft3. (a) Is the density of water greater than the density ...
... 1.81 Antifreeze contains the compound ethylene glycol. This compound not only lowers the freezing point of water but also increases the boiling point of water. The density of ethylene glycol is 9.35 lb/gal, and the density of water is 62.5 lb/ft3. (a) Is the density of water greater than the density ...
Alternative Coverage of moles, molarity, and Chemical Calculations
... Like atomic masses, molecular masses are relative masses. A molecule of oxygen, O2, has a mass of 32 u, twice that of a molecule of methane, 16 u. A molecule of ozone has a mass of 48 u, three times that of a molecule of methane. Using the same reasoning we used for atomic substances, we conclude th ...
... Like atomic masses, molecular masses are relative masses. A molecule of oxygen, O2, has a mass of 32 u, twice that of a molecule of methane, 16 u. A molecule of ozone has a mass of 48 u, three times that of a molecule of methane. Using the same reasoning we used for atomic substances, we conclude th ...
Possible pieces of introduction:
... Here Levi admits that not only has chemistry as a trade been a large part of his life in which he has experienced many things, but it has also allowed him the solace of understanding that all men are created equal on some level. His understanding of the chemistry of life, the way that carbon will en ...
... Here Levi admits that not only has chemistry as a trade been a large part of his life in which he has experienced many things, but it has also allowed him the solace of understanding that all men are created equal on some level. His understanding of the chemistry of life, the way that carbon will en ...
Benzylamine reacts with nitrous acid to form unstable
... Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule. Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement o ...
... Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule. Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement o ...
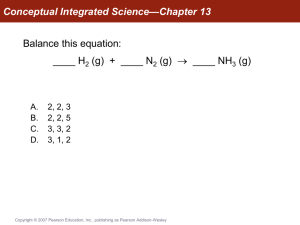
Conceptual Integrated Science—Chapter 13
... The warm air from a lit birthday candle does not rise within an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the ca ...
... The warm air from a lit birthday candle does not rise within an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the ca ...
To do List
... The element samarium is known to have three isotopes - Sm-148, Sm-149, and Sm-152. The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abund ...
... The element samarium is known to have three isotopes - Sm-148, Sm-149, and Sm-152. The masses of these three isotopes are 148.1010 amu, 149.2005 amu, and 152.4107 amu, respectively. If the lightest isotope is three times as abundant as the heaviest, and the middle isotope is known to be 16.00% abund ...
Higher Chemistry Resources Guide - Glow Blogs
... Learners should encounter covalent molecular compounds that contain a metal. Tin(IV) iodide can be formed by gently heating tin and iodine in toluene in a small conical flask. When the mixture is allowed to cool, yellow-brown crystals form which can be collected by filtration. Melting point of SnI4 ...
... Learners should encounter covalent molecular compounds that contain a metal. Tin(IV) iodide can be formed by gently heating tin and iodine in toluene in a small conical flask. When the mixture is allowed to cool, yellow-brown crystals form which can be collected by filtration. Melting point of SnI4 ...
Curriculum Vitae - Université Paris-Sud
... Université Paris-Sud, 91405 Orsay, France For more than two decades, extensive research work has been devoted to the unique properties of clusters. They are made of a small number (or nuclearity) of atoms or molecules only, and therefore constitute a new state of matter, or mesoscopic phase, between ...
... Université Paris-Sud, 91405 Orsay, France For more than two decades, extensive research work has been devoted to the unique properties of clusters. They are made of a small number (or nuclearity) of atoms or molecules only, and therefore constitute a new state of matter, or mesoscopic phase, between ...
PDF of this page
... contextual problems as a means to explore introductory models and concepts of chemistry. Students will also gain an understanding of how scientific models are used to explain experimental observations. The laboratory component of this course is designed to provide students with an experimental conte ...
... contextual problems as a means to explore introductory models and concepts of chemistry. Students will also gain an understanding of how scientific models are used to explain experimental observations. The laboratory component of this course is designed to provide students with an experimental conte ...
CHAPTER-8 NCERT SOLUTIONS
... HI and HBr can reduce H2SO4 to SO2, but HCl and HF cannot. Therefore, HI and HBr are stronger reductants than HCl and HF. ...
... HI and HBr can reduce H2SO4 to SO2, but HCl and HF cannot. Therefore, HI and HBr are stronger reductants than HCl and HF. ...
Answers - logo Pre-U Chemistry Textbook
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
Changing Matter
... Making a mixture – 2 or more types of matter (substances) mixed together • Not in specific amounts • Can be separated physically ...
... Making a mixture – 2 or more types of matter (substances) mixed together • Not in specific amounts • Can be separated physically ...
Exam Review
... 19. Draw Lewis Structures, sketch and name the shape of the molecule, predict the bond polarity, and molecule polarity for the following molecules: a) PH3 ...
... 19. Draw Lewis Structures, sketch and name the shape of the molecule, predict the bond polarity, and molecule polarity for the following molecules: a) PH3 ...
Semester 4 - Vaal University of Technology
... WIL is an integral part of the training and, together with University Training, form a co-operative training unit. It is therefore the aim of WIL to compel the students in his/her work situation, to be actively engaged in the broadening of his/her knowledge and analytical skills. It is also importan ...
... WIL is an integral part of the training and, together with University Training, form a co-operative training unit. It is therefore the aim of WIL to compel the students in his/her work situation, to be actively engaged in the broadening of his/her knowledge and analytical skills. It is also importan ...
Chapter 3
... One of cancer chemotherapy’s greatest success stories began with an accidental discovery. In 1964, Barnett Rosenberg and his research group at Michigan State University were studying the effect of an electric field on the growth of bacteria. Using platinum electrodes, they passed an electric current ...
... One of cancer chemotherapy’s greatest success stories began with an accidental discovery. In 1964, Barnett Rosenberg and his research group at Michigan State University were studying the effect of an electric field on the growth of bacteria. Using platinum electrodes, they passed an electric current ...
Physics Jeopardy! - The Pingry School
... This postulate of Dalton’s was disproved with the discovery of subatomic particles back ...
... This postulate of Dalton’s was disproved with the discovery of subatomic particles back ...
Study Guide for Chapter 22 - Hydrocarbon Compounds
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
Higher Chemistry Resources Guide - Glow Blogs
... Learners should encounter covalent molecular compounds that contain a metal. Tin(IV) iodide can be formed by gently heating tin and iodine in toluene in a small conical flask. When the mixture is allowed to cool, yellow-brown crystals form which can be collected by filtration. Melting point of SnI4 ...
... Learners should encounter covalent molecular compounds that contain a metal. Tin(IV) iodide can be formed by gently heating tin and iodine in toluene in a small conical flask. When the mixture is allowed to cool, yellow-brown crystals form which can be collected by filtration. Melting point of SnI4 ...
Chapter 3 Lectures
... a) the mass of tin produced from 0.211 moles of hydrogen gas. b) the number of moles of H2O produced from 339 grams of SnO2. c) the mass of SnO2 required to produce 39.4 grams of tin. d) the number of atoms of tin produced in the reaction of 3.00 grams of H2. e) the mass of SnO2 required to produce ...
... a) the mass of tin produced from 0.211 moles of hydrogen gas. b) the number of moles of H2O produced from 339 grams of SnO2. c) the mass of SnO2 required to produce 39.4 grams of tin. d) the number of atoms of tin produced in the reaction of 3.00 grams of H2. e) the mass of SnO2 required to produce ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.