Unit 2 Notes unit_2_atomic-nuclear-electronic
... • Two elements or more elements may form more than one compound if they have different whole number ratio of each element. • Example: water H2O hydrogen peroxide ...
... • Two elements or more elements may form more than one compound if they have different whole number ratio of each element. • Example: water H2O hydrogen peroxide ...
atom - Huntington Catholic School
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
Chemistry Unit 2 - Finding Patterns
... Atomic theory has been built on the measurements, observations, discoveries, and interpretations of data by many scientists, and has been modified over the centuries by both major discoveries and small modifications so that our current understanding of atomic structure is best explained by the wave- ...
... Atomic theory has been built on the measurements, observations, discoveries, and interpretations of data by many scientists, and has been modified over the centuries by both major discoveries and small modifications so that our current understanding of atomic structure is best explained by the wave- ...
01 Atomic Structure.p65
... molecules are bombarded by these electrons. The collisions cause electrons to be removed from the atoms/molecules of the sample, so positive ions are formed. (This is where fragmentation can occur – ...
... molecules are bombarded by these electrons. The collisions cause electrons to be removed from the atoms/molecules of the sample, so positive ions are formed. (This is where fragmentation can occur – ...
CMC Chapter 5
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
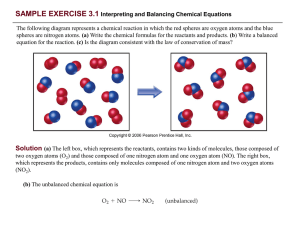
PRACTICE EXERCISE - Needham.K12.ma.us
... Analyze: We are given the number of grams and chemical formula and asked to calculate (a) the number of molecules and (b) the number of O atoms in the sample. (a) Plan: The strategy for determining the number of molecules in a given quantity of a substance is summarized in Figure 3.10. We must conve ...
... Analyze: We are given the number of grams and chemical formula and asked to calculate (a) the number of molecules and (b) the number of O atoms in the sample. (a) Plan: The strategy for determining the number of molecules in a given quantity of a substance is summarized in Figure 3.10. We must conve ...
Name
... 18. Sulfur in gasoline can produce sulfuric acid, H2SO4, according to the two-step process shown below. For each 125g of sulfur in gasoline, how many moles of H2SO4 will be produced? S(s) + O2(g) → SO2(g) 2SO2(g) + 2H2O(l) + O2(g) → 2H2SO4(aq) a. 4.5mol b. 2.1mol c. 3.9mol d. 1.4mol 19. How many at ...
... 18. Sulfur in gasoline can produce sulfuric acid, H2SO4, according to the two-step process shown below. For each 125g of sulfur in gasoline, how many moles of H2SO4 will be produced? S(s) + O2(g) → SO2(g) 2SO2(g) + 2H2O(l) + O2(g) → 2H2SO4(aq) a. 4.5mol b. 2.1mol c. 3.9mol d. 1.4mol 19. How many at ...
Slide 1
... oxygen. THIS MEANS THAT IF YOU HAD 100 grams, YOU WOULD HAVE 40.00 g CARBON 6.72 g HYDROGEN 53.8 g OXYGEN ...
... oxygen. THIS MEANS THAT IF YOU HAD 100 grams, YOU WOULD HAVE 40.00 g CARBON 6.72 g HYDROGEN 53.8 g OXYGEN ...
Hybridization
... Lewis structures and VSEPR are useful tools for predicting the shape of a molecule or ion, but they really do not provide any information about the bonds that exist between the atoms; they do not tell us why covalent bonds form nor do they describe what happens to the atomic orbitals when the bond f ...
... Lewis structures and VSEPR are useful tools for predicting the shape of a molecule or ion, but they really do not provide any information about the bonds that exist between the atoms; they do not tell us why covalent bonds form nor do they describe what happens to the atomic orbitals when the bond f ...
Drawing Atomic Structure
... An atom is the smallest particle of an element that still retains the properties of that element. Atomic Symbol - The shorthand abbreviation that is used to identify an element (element name) - Atomic symbols have o A capital letter at the beginning of the symbol (all symbols have this) o Some have ...
... An atom is the smallest particle of an element that still retains the properties of that element. Atomic Symbol - The shorthand abbreviation that is used to identify an element (element name) - Atomic symbols have o A capital letter at the beginning of the symbol (all symbols have this) o Some have ...
Isotopes Article
... What is an ISOTOPE? We all know what an atom is by now and we are aware that all matter is made up of them. Atoms themselves are made up of three subatomic particles: protons, neutrons, and electrons. Each of those has different charges. The protons (positive) and neutrons (no charge) are found in t ...
... What is an ISOTOPE? We all know what an atom is by now and we are aware that all matter is made up of them. Atoms themselves are made up of three subatomic particles: protons, neutrons, and electrons. Each of those has different charges. The protons (positive) and neutrons (no charge) are found in t ...
Atoms and Molecules
... an ancient Greek word that means “uncuttable.” Democritus could not see an atom (as we can today), but he had figured out something very important. His atom is what we talk about today as an element. Q: Give an example of an element. A: Answers will vary; Examples include hydrogen, oxygen, gold, etc ...
... an ancient Greek word that means “uncuttable.” Democritus could not see an atom (as we can today), but he had figured out something very important. His atom is what we talk about today as an element. Q: Give an example of an element. A: Answers will vary; Examples include hydrogen, oxygen, gold, etc ...
ionic compound
... A Lithium ION, however, results when the atom loses the single electron in the outer most level during a chemical reaction with the fluorine. The fluorine ION forms when it gains the electron lost by the lithium. The lithium is now equivalent to Helium and the Fluorine to Neon in terms of the number ...
... A Lithium ION, however, results when the atom loses the single electron in the outer most level during a chemical reaction with the fluorine. The fluorine ION forms when it gains the electron lost by the lithium. The lithium is now equivalent to Helium and the Fluorine to Neon in terms of the number ...
Ionization energy
... - react with water to form hydrogen and alkaline solutions; burn in air - al-quili means wood ashes - term dates back to ancient times; people discovered that wood ashes mix with water to produce slippery solutions that can remove grease - one outer electron, by losing this electron they become a ca ...
... - react with water to form hydrogen and alkaline solutions; burn in air - al-quili means wood ashes - term dates back to ancient times; people discovered that wood ashes mix with water to produce slippery solutions that can remove grease - one outer electron, by losing this electron they become a ca ...
Chemical Equilibrium
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
Unit 6 Regents Level
... (1) This could be due to _____________________________________________ (2) This unstable state is called the ______________________________ ii) After the electron quickly returns to the _______________________, it ____________ the same amount of energy it absorbed. (1) The energy given off is ______ ...
... (1) This could be due to _____________________________________________ (2) This unstable state is called the ______________________________ ii) After the electron quickly returns to the _______________________, it ____________ the same amount of energy it absorbed. (1) The energy given off is ______ ...
Unit 2 - The Atom 1-3.key
... this information, Which isotope on of hydrogen must be the mostwhich abundant, based on this data? ____ isotope must be the most abundant? ...
... this information, Which isotope on of hydrogen must be the mostwhich abundant, based on this data? ____ isotope must be the most abundant? ...
Elements and Compounds
... Chemical and Physical Properties • Elements • An Element is the basic form of matter – it cannot be broken down into smaller parts by chemical methods Each element has a unique identity ...
... Chemical and Physical Properties • Elements • An Element is the basic form of matter – it cannot be broken down into smaller parts by chemical methods Each element has a unique identity ...
File - GarzScience!
... • It could act as both particles and waves • Bohr proposed a model that explained why only certain frequencies of light were emitted from hydrogen. ...
... • It could act as both particles and waves • Bohr proposed a model that explained why only certain frequencies of light were emitted from hydrogen. ...
4.2 Discovering Parts of the Atom
... • Rutherford also discovered the proton, a particle with a positive charge. • Rutherford knew the mass of a proton, but could not account for the total mass of an atom. • Rutherford’s theory was later confirmed when the existence of the neutron—a neutral atomic particle with a mass similar to a prot ...
... • Rutherford also discovered the proton, a particle with a positive charge. • Rutherford knew the mass of a proton, but could not account for the total mass of an atom. • Rutherford’s theory was later confirmed when the existence of the neutron—a neutral atomic particle with a mass similar to a prot ...
23.32 KB - KFUPM Resources v3
... A) The hydrogen atom has only one orbital. B) The size of the hydrogen 1s orbital is defined as the surface that contains 90% of the total electron probability. C) The square of the wave function represents the probability distribution of the elctron in the orbital. D) In the quantum mechanical mode ...
... A) The hydrogen atom has only one orbital. B) The size of the hydrogen 1s orbital is defined as the surface that contains 90% of the total electron probability. C) The square of the wave function represents the probability distribution of the elctron in the orbital. D) In the quantum mechanical mode ...
MOLES AND CALCULATIONS USING THE MOLE CONCEPT
... 1. A mole is the amount of any substance that contains as many elementary entities as there are atoms in exactly 1.00 g of hydrogen-1. 2. A mole is the amount ... in exactly 12.00 g of carbon-12. 3. 6.02 x 1023 of anything 4. It is important to state the entities involved: atoms, molecules, ions, el ...
... 1. A mole is the amount of any substance that contains as many elementary entities as there are atoms in exactly 1.00 g of hydrogen-1. 2. A mole is the amount ... in exactly 12.00 g of carbon-12. 3. 6.02 x 1023 of anything 4. It is important to state the entities involved: atoms, molecules, ions, el ...
TEST-Atomic Structure
... b. Unlike protons or neutrons, electrons have no mass. c. Neutrons have no charge and no mass. d. An electron has far less mass than either a proton or neutron. ____ 15. Which of the following is unique for any given element? a. the number of neutrons c. the number of protons b. the charge on the el ...
... b. Unlike protons or neutrons, electrons have no mass. c. Neutrons have no charge and no mass. d. An electron has far less mass than either a proton or neutron. ____ 15. Which of the following is unique for any given element? a. the number of neutrons c. the number of protons b. the charge on the el ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.